All done here! Check out our other subjects!
Abstract
- Breast cancer occurs in approximately one in every seven women and is usually an adenocarcinoma.
- The risk of developing cancer increases with exposure to oestrogen throughout the woman’s life and if mutations in certain tumour suppressor genes are present.
- Breast lumps are investigated using the triple assessment technique consisting of clinical history and examination, imaging and histological examination.
- Breast cancers can be classified into either non-invasive or invasive and by their molecular classification.
- The treatment of primary breast cancer involves mainly surgical resection with adjunct medical treatment alongside.
Core
Breast cancer is the most common cancer in the UK, accounting for more than 15% of all newly diagnosed cancers in the UK and for more than 20% of female malignancies. Approximately one in every seven women will develop breast cancer in their lifetime. Over 95% of malignant neoplasms in the breast are adenocarcinomas[1], due to the highly glandular structure of the breast.
The largest risk factors of breast cancer are female sex and older age.
Risk is also associated with increased exposure to oestrogen. The cells of the breast glandular tissue have hormone receptors on their cell surface that allow them to respond accordingly to different levels of hormones, specifically oestrogen, progesterone and prolactin. When oestrogen is higher, the glandular tissue proliferates. Milk production itself is driven mainly by prolactin rather than oestrogen. With increased division and proliferation of these cells, there is a higher chance of an error occurring and uncontrolled cell growth as a result.
Things which increase oestrogen exposure include: female sex, early menarche (first period below the age of 11), late menopause, late first pregnancy (over age 35) and nulliparity (never having children).
Exogenous oestrogens (those which have their origin outside the body) from hormone replacement therapies are known to slightly increase the risk.
Other strong risk factors for malignant breast disease are if the patient has had previous breast cancer, atypical changes on a previous biopsy, any previous cancers, any exposure to radiation and a family history of breast cancer.
Autosomal dominant mutations in tumour suppressor genes, BRCA1 (breast cancer associated gene 1) & BRCA2, are the known cause of 25% of hereditary breast cancer cases but only 2% of all breast cancers. Carriers of these mutations will present with symptoms earlier in life and have a very high likelihood of developing breast cancer in their lifetime, so much so that some carriers choose to undergo prophylactic double mastectomies (removal of both breasts to prevent them from developing any cancer).
Male breast cancer is far rarer, comprising only 1% of all breast cancer diagnoses. Specific risks include Klinefelter syndrome, where the male has an additional X chromosome thus a genotype of 47, XXY. Again, just as in females, the risk is related to oestrogen exposure and increased with BRCA gene mutations.
Obesity increases the risk of breast cancer as endogenous androgens are converted into oestrogen in any peripherally stored fat by the enzyme aromatase. The more fat present, the higher the conversion, production and exposure to oestrogens.
Breast feeding is known to be protective against breast cancer as lactation suppresses ovulation and so exposure of oestrogen to the luminal cells of the breast is reduced.
If a GP suspects that a breast mass may be cancerous, the patient is referred on to a two-week wait pathway to a clinic run by breast surgeons. The mass is then investigated using the triple assessment technique, a universal three-part process involving clinical history and examination, radiographic imaging and histological examination.
- The surgeon will take an in-depth history about the presentation of the breast lump, asking questions about; when and how it was noticed, how it feels to the patient, if the lump has changed, if the patient noticed any other changes in themselves - specifically any concerning features. Systemic symptoms such as tiredness, unexplained weight loss or night sweats are non-specific and are uncommon in early breast cancer. and has the patient got any of the aforementioned risk factors.
- After a thorough history, the surgeon will then examine the lump. They will be looking at the surrounding skin for any signs, the location of the lump (malignancies often present in the upper outer quadrant of the breast), the texture of the lump, if the lump is mobile or tethered to one spot and if there is any lymph node involvement in the axilla.
- After the consultation the patient will then be taken for radiographic imaging of the lump, either by mammogram (see benign breast disease article) or ultrasound scanning. This will assist in differentiating any abnormal changes compared to the normal breast tissue, and identify the nature of this mass.
- The surgeon or radiologist may then take a sample of the tissue for review by a histopathologist to look for any cell changes. The sample will either be taken as a biopsy or a fine needle aspiration of the tumour and reviewed.

Diagram - A biopsy of a breast mass for histological analysis
Creative commons source by BruceBlaus [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
Around 95% of breast carcinomas are adenocarcinomas, which are then classified in two different ways, by the structure of origin and the metastatic potential (in situ or invasive). An In-situ carcinoma describes when the neoplasm is restrained by the basement membrane so is contained within the lobules or ducts, and has not invaded into other tissues. Invasive carcinomas are those which have broken through into other tissues and are able to metastasise as a result.
There are two main types of non-invasive breast carcinomas named after the structure of origin, Ductal Carcinoma In Situ (DCIS) and Lobular Carcinoma In Situ (LCIS).
Ductal Carcinoma In Situ is a malignancy originating from the epithelial cells lining the lactiferous ducts, with an intact myoepithelial layer and basement membrane. DCIS is usually asymptomatic and is detected incidentally through the NHS breast screening programme. DCIS has the potential to progress to invasive cancer, and therefore is usually surgically removed either as a lumpectomy (wide local excision) or mastectomy.
Lobular Carcinoma In Situ is less common. It originates from the secretory lobules of the breast which but has not invaded into surrounding tissue. It is often diagnosed as an incidental finding during biopsy as is mostly asymptomatic. LCIS is considered a marker of increased risk of developing invasive breast cancer in either breast rather than a direct precursor lesion, and so observation alone is often possible rather than surgical resection.
Paget’s disease of the nipple is an unusual presentation which is nearly always associated with an underlying neoplasm. Paget’s is characterised by the development of a red, scaly rash of the nipple and areola. There may be an itchy or burning sensation associated with pain and possible abnormal discharge from the nipple. The nipple may also become inverted.
Paget’s disease of the breast is caused by malignant cells spreading along the duct to involve the nipple epidermis, and is usually associated with an underlying ductal carcinoma in situ or invasive carcinoma. It is easily mistaken for dermatitis or eczema, and so this presentation should be treated with high suspicion and referred to a specialist. Treatment depends on the underlying neoplasm, but both the nipple and areolar will require surgical excision.
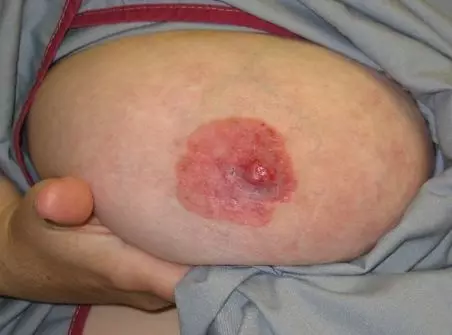
Image - The reddening and ulceration of the nipple associated with Paget’s disease
Public Domain Source by Lily Chu, National Naval Medical Center Bethesda [Public domain]
Carcinomas become invasive when they have broken through the basement membrane into the stroma and surrounding structures of the breast. As a result, these tumours can potentially metastasise through either lymphatic drainage or blood vessels. The primary point of metastasis is often to the lymph nodes in the ipsilateral axilla. The first lymph node that a given area of the breast drains to is known as the sentinel lymph node and so, if any, is the most likely lymph node to contain metastases. Distant metastasis sites which include the lungs, liver, brain or most commonly bone are accessed via the blood vessels.
Invasive carcinoma of the breast is classified by the structure of origin, with invasive ductal carcinoma (also known as No Special Type - NST) by far being the most common generating around 70% of invasive breast malignancy diagnoses. The structure of the invasive ductal carcinoma depends on how differentiated the tumour is, a well differentiated neoplasm consists of tubules lined by atypical cells, whereas a poorly differentiated neoplasm consists of sheets of pleomorphic cells (lots of variability in size, shape and type). This often develops from DCIS tumours that have not been caught or treated.
Invasive lobular carcinoma originates from the secretory lobules of the breast and is the second most common breast cancer. It generates around 15% of invasive breast malignancy diagnoses.
As well as mutations in tumour suppression genes BRCA 1 and 2 as mentioned previously, some breast cancers can have amplification of the Human Epidermal Growth Factor Receptor 2 gene, which leads to increased production of the HER2 receptor. The normal action of this receptor is to stimulate cell growth. Mutations in this gene can cause an overexpression in these surface proteins and contribute to the uncontrolled proliferation of cells. HER2 positive cells are those that have an increased amount of this receptor protein on their surface compared to normal HER2 negative cells. Oestrogen receptors are also key in breast cancers for their role in cell proliferation as described above.
Like the glandular cells they develop from, the breast cancer cells can have hormone receptors on the surface. As a result, the tumour can be classified not just by location and invasiveness but also by the different receptors that are present on its surface. Knowing the expression of certain receptors allows for treatment to be tailored to that specific tumour. There are three major types:
- ER positive (oestrogen receptor labelled as ER from the American spelling of Estrogen) and HER2 negative carcinoma. This is by far the most common pattern and has the best prognosis.
- HER2 positive and ER positive/negative carcinoma
- HER2 negative and ER negative carcinoma ‘triple negative’ cancer (taking into account progesterone receptors).
Around 20% of breast cancers are HER2 positive, they are more aggressive and have a higher recurrence rate than HER2 negative, but drugs such as Herceptin can be used to specifically target these cells. ER positive breast cancers can be targeted using drugs such as Tamoxifen.
Lymphatic vessels can become blocked through either compression from a tumour or a neoplasm invading the vessel walls. This can cause issues with the drainage of fluid from the breast and so leads to swelling, presenting as the clinical sign of peau d’orange discussed in the previous article.
The local inflammation that is responding to the tumour can lead to fibrosis of the suspensory ligaments and the lactiferous ducts, reducing the milk secretory function of the breast.
Tumours can metastasise to various points around the body causing different effects depending on that location.
Management of Primary Breast Cancer
The mainstay of primary breast cancer treatment is surgery to remove the tumour, and then additional adjuvant treatments such as chemotherapy, radiotherapy and hormonal therapy to increase the overall effectiveness of the treatment. The level of treatment depends on the type, stage and grade of the cancer.
Breast surgery involves removing the tumour. This can be done as a lumpectomy; removal of the tumour using a wide excision of the surrounding breast tissue to prevent any cancer from remaining in the breast. This technique tends to be done on smaller tumours. For larger tumours, a mastectomy (removal of the entire breast) may be required. These are sometimes done prophylactically on women who carry mutations in BRCA1 and BRCA2 genes to reduce the chance of breast cancer.
Sentinel node biopsies are often done during breast surgery. This involves injecting a blue dye and or a radioactive tracer into the tumour or surrounding tissue and then tracking which lymph nodes it drains to first. The lymph nodes can be detected using a Geiger counter and biopsied for histological assessment. If they contain metastasis then lymph node clearance has to be undertaken. This involves dissecting the axilla and removing all the lymph nodes without damaging any of the important structures. Complications include the development of lymphoedema and seroma formation due to the reduced drainage of the area, and rarely neurological damage if any of the brachial plexus becomes injured during the surgery.
Medical treatment is often used to reduce the risk of recurrence of breast cancer post-surgery or as the mainstay of treatment for those unfit for surgery. Alongside chemotherapy and radiotherapy, hormonal therapies and immunotherapies are very commonly used in breast cancer management.
- Tamoxifen is an anti-oestrogen that can be used to target ER positive breast cancers. It binds to the oestrogen receptors and inhibits the proliferative oestrogen stimulus on the tumour and so reducing any further malignant growth.
- Aromatase inhibitors such as anastrozole also deprive the cancer cells of oestrogen stimulus but by a different mechanism. They reduce the peripheral conversion of androgens to oestrogen, and so are commonly used in post-menopausal women who have no other supply of oestrogen.
- Targeted biological medications like Herceptin (trastuzumab), a monoclonal antibody that targets the receptors on HER2 positive cancers, blocking them from receiving growth signals and so, restricting the growth of the cancer. These antibodies can also encourage the body's immune system to target and destroy the cancerous cells to which they have bound.
We use Cancer Research UK’s statistics throughout this article https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero
Any specific references can be sourced below.
[1] https://training.seer.cancer.gov/breast/intro/types.html
Edited by: Dr. Christopher Bradley MD, FRCP, Consultant Oncologist
Edited by: Dr. Ben Appleby
Review by: Dr. Thomas Burnell
Reviewed by: Dr. Marcus Judge
- 3569

