Next Lesson - Biological Membranes
Contents
- Protein targeting
- Overview of cellular protein transportation system
- Proteins targeted to a cell organelle
- Proteins targeted to the nucleus
- Proteins targeted to the mitochondria
- Proteins targeted to peroxisomes
- Proteins targeted to the endoplasmic reticulum and secretory pathway
- Proteins targeted to the lysosomes
- Proteins that remain in the endoplasmic reticulum
- Proteins targeted to the plasma membrane and secretion
- Quiz
Abstract
- For proteins to carry out their function in the correct part of the cell or be secreted out of the cell, the polypeptide must contain an address label or signal sequence so that the molecule can be delivered to the correct destination.
- Proteins destined for the cytoplasm or organelles such as the nucleus or peroxisomes, are produced at free ribosomes.
- Proteins destined for the plasma membrane or secretion out of the cell are produced at the ribosomes attached to endoplasmic reticulum.
- The signal sequence is recognised by a receptor and forms a complex, which is transported to the correct organelle. At the organelle, there are mechanisms that aid the transport of the protein to the organelle and energy is required at some point in the process.
- If a protein is destined for the endoplasmic reticulum membrane or the plasma membrane, there is an address label or another sequence of amino acids in the polypeptide chain that will lead the protein to the membrane of an organelle or the plasma membrane.
- Some proteins must remain in the endoplasmic reticulum or the Golgi apparatus and will contain a specific tag in the sequence of amino acids that allows the protein to remain in one of these organelles and not be transported to another one.
Core
Proteins need to be sent to the correct organelle within the cell or sometimes need to be exported out of the cell into the extracellular space to carry out their function. To make sure the proteins arrive at the correct destination, the polypeptide will have a molecular label (specific amino acid sequence) that will ensure the protein is delivered to a specific location. Within the cell itself, there are various systems that help to deliver the proteins, produced in the process of translation, to reach their end terminus.
Overview of Cellular Protein Transportation Systems
- Proteins intended for the cytoplasm or the post-translational delivery to organelles such as the nucleus or peroxisome are produced on free ribosomes.
- Proteins intended for the membrane or the exterior of the cell are produced on ribosomes attached to the rough endoplasmic reticulum (RER).
At each stage of the process of the translation, the protein being produced is being checked for molecular signals to see if the protein needs to be transported to a different site within the cell to carry on its production or to see if the overall destination of the protein has changed.
Proteins Targeted to a Cell Organelle
Proteins that are destined for organelles (e.g. the nucleus, mitochondria, peroxisomes) or the cytosol are firstly produced in the cytoplasm then delivered to the relative destination.
To be delivered to one of the organelles mentioned, the protein will contain a signal sequence or address label that is recognised by a receptor, which then directs it to the correct organelle. Once at the organelle, there are mechanisms that aid the protein across the membrane of the organelle and in the process of delivering the protein to its final destination, energy can be transferred to continue the process of transporting proteins.
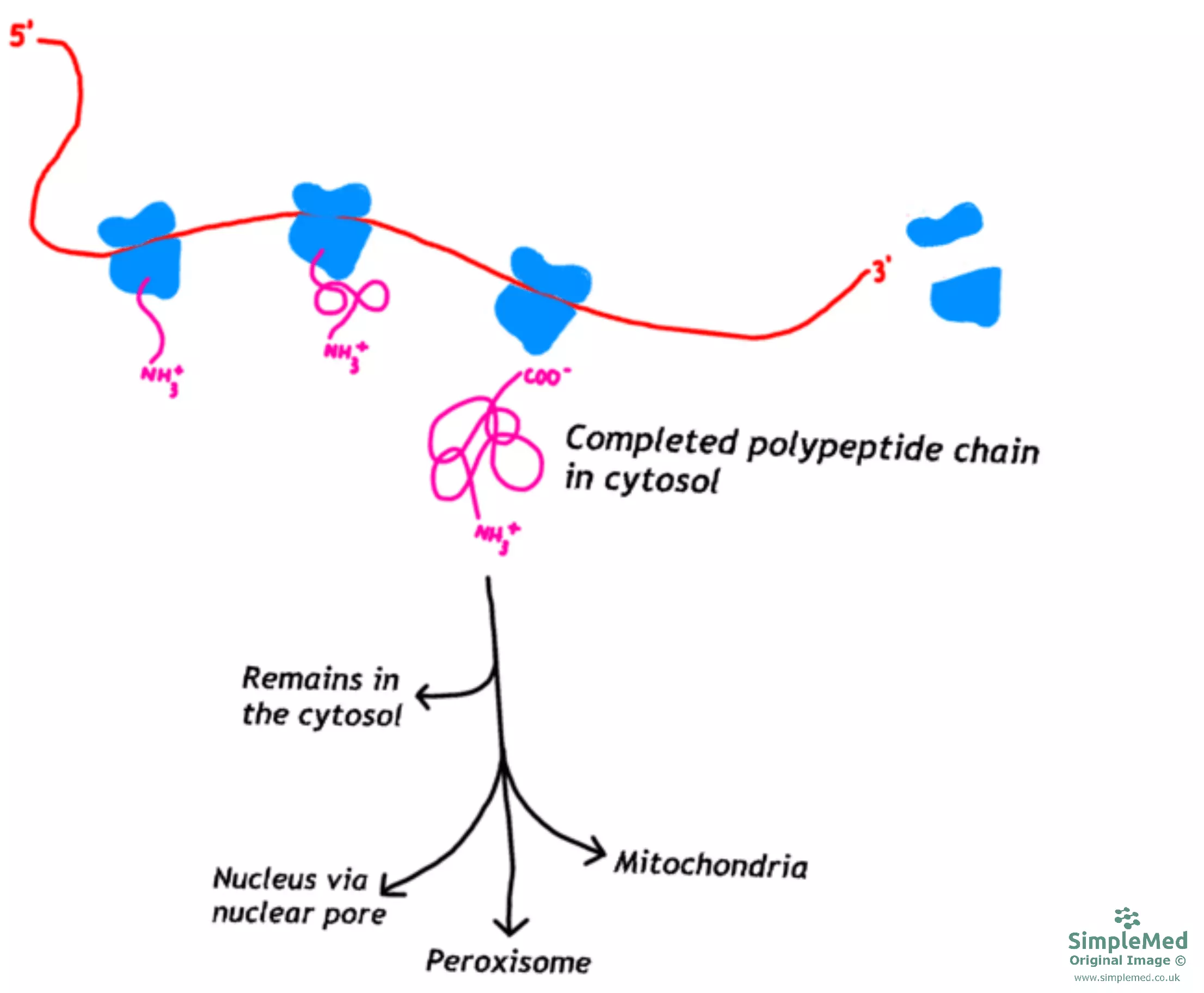
Diagram - The pathway for proteins targeted for organelles or the cytosol
SimpleMed original by Dr. Peter Parkinson
Proteins Targeted to the Nucleus
The nucleus has many proteins that are involved in the biological processes that the nucleus undertakes, however, all the proteins found in the nucleus are produced in the cytoplasm, so they need to be transported to the nucleus.
For the proteins to enter the nucleus, they have pass through a protein channel in the nuclear membrane called a nuclear pore. Proteins targeted for the nucleus will contain a nuclear localisation sequence (NLS), which is a run of 4-8 basic amino acids in the primary sequence of the polypeptide chain.
A folded protein with the NLS sequence enters the nucleus from the cytosol with the help of other proteins called importins. The protein with the NLS sequence and importins form a complex, which then binds to the nuclear pore and is transported into the nucleus using energy. When inside the nucleus, the nuclear protein separates from the complex and the importins then bind to a GTPase protein (Ran). The importins are released out of the nucleus along with Ran, so the importins can be reused to transport more proteins into the nucleus, while Ran is recycled to maintain the direction of nuclear transport through its GTP-bound and GDP-bound states.
Proteins Targeted to Mitochondria
Proteins destined for the mitochondria are produced in the cytoplasm in an unfolded state, with the address label being an amphipathic sequence made up of 10-80 amino acids found at the N-terminal. The unfolded protein is stabilised by interacting with molecular chaperones in the cytoplasm.
At the mitochondrial outer membrane, the signal sequence is recognised and a protein channel forms to allow the transport of the protein through the outer mitochondrial membrane. These channels are called translocase of the outer membrane or TOM.
If the protein is destined for the matrix of the mitochondria, the protein is transported through the inner mitochondrial membrane via another protein channel called translocase of the inner membrane or TIM. This process requires a membrane potential and energy.
If the protein is required within the structure of the inner mitochondrial membrane, there will additional signals within the primary sequence of the protein to halt the protein from entering the matrix of the mitochondria.
When the protein has crossed the inner mitochondrial membrane, an enzyme called mitochondrial processing peptidase will remove the signal sequence from the protein. The protein will then fold into its conformation in a process requiring chaperone proteins and energy.
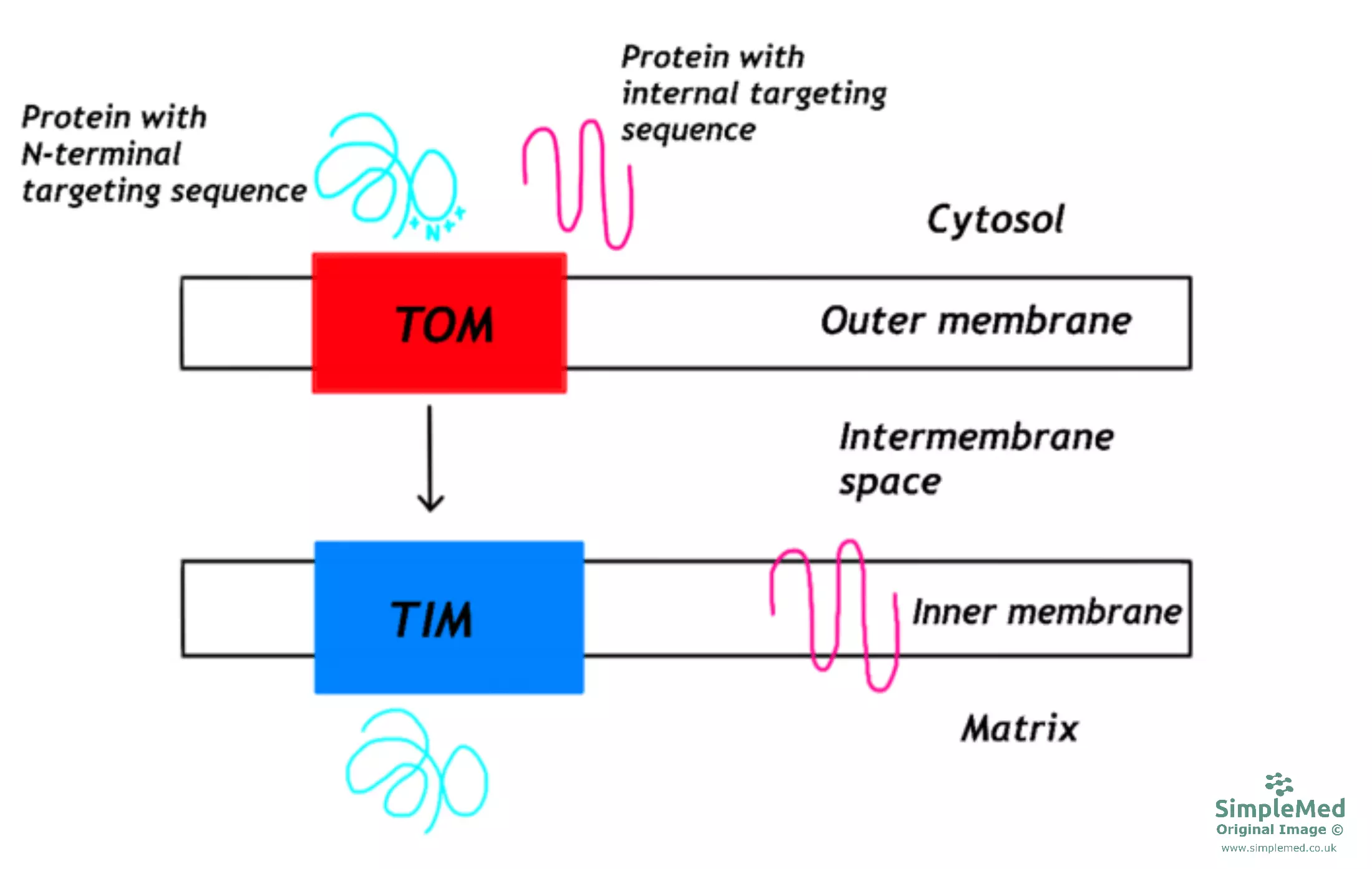
Diagram - The pathway for proteins targeted for mitochondria
SimpleMed original by Dr. Peter Parkinson
Proteins Targeted to Peroxisomes
Peroxisomes have a role in the cell by metabolising lipids and detoxifying chemical molecules.
Proteins targeted for peroxisomes are first produced in the cytosol and require a peroxisome targeting sequence (PTS) to direct them to peroxisomes. The PTS is the address label and the address label is formed of a run of three amino acids: serine-lysine-leucine. The address for proteins destined for peroxisomes is found at the C-terminal of the polypeptide chain. The receptor that recognises the signal is the PTS receptor, which binds to the protein in the cytoplasm of the cell.
Within the peroxisome membrane, there is a protein channel that the PTS receptor-protein complex binds to, and then the protein channel opens to allow the passage of the protein into the matrix of the peroxisome. Once in the matrix of the peroxisome, the PTS sequence remains attached to the polypeptide chain and the PTS receptor is returned to the cytosol, requiring energy by hydrolysing ATP.
Proteins Targeted to the Endoplasmic Reticulum and Secretory Pathway
When a protein is intended for any part of the endomembrane system (endoplasmic reticulum, Golgi apparatus, lysosome and plasma membrane) or secretion out of the cell, the polypeptide is firstly taken to the endoplasmic reticulum (ER) during translation.
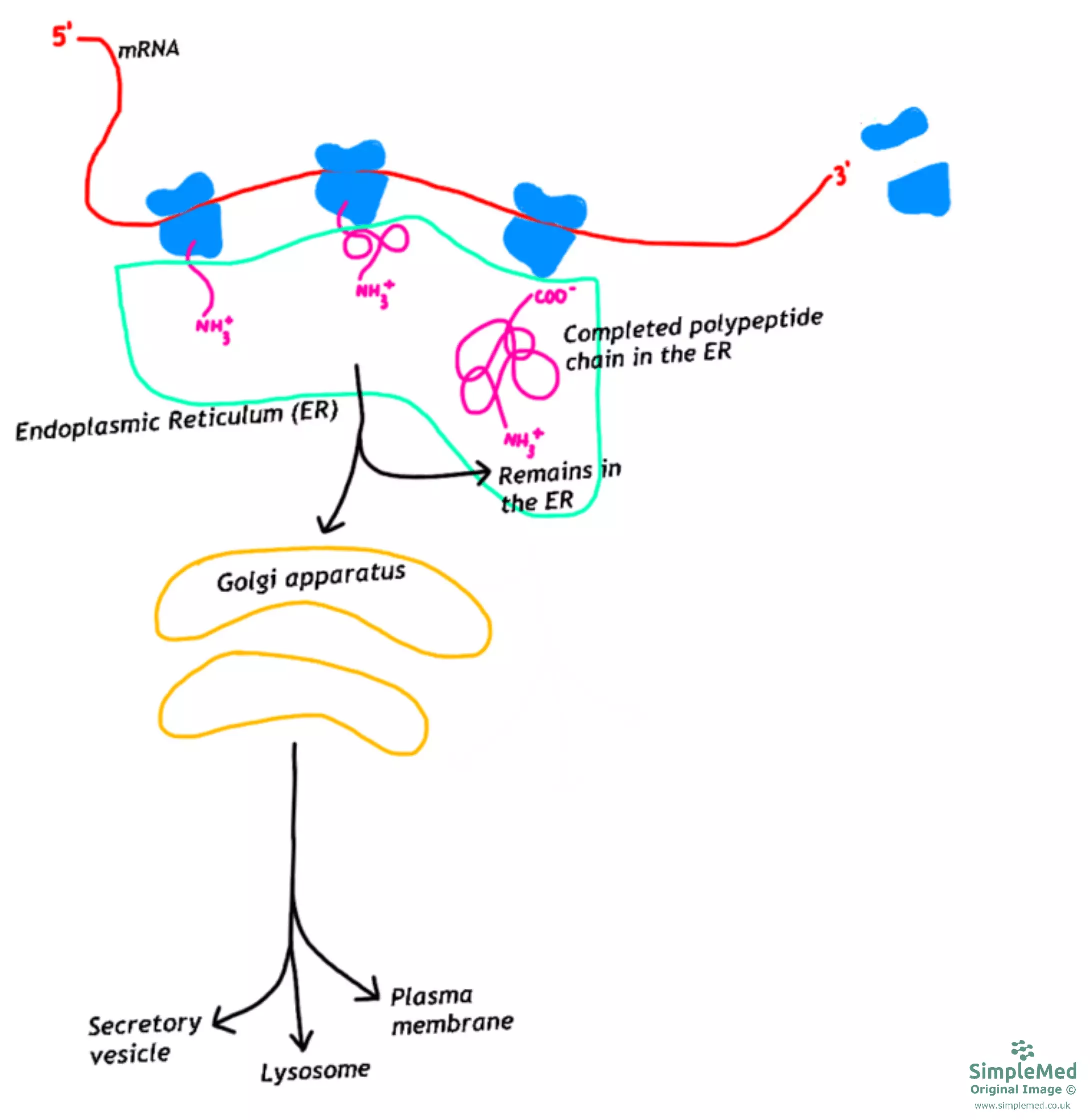
Diagram - The pathway for proteins targeted for secretion and the endoplasmic reticulum
SimpleMed original by Dr. Peter Parkinson
For a protein to be transported to the ER, the polypeptide chain must have a signal sequence, which is a run of hydrophobic amino acids found at the N-terminal of the chain.
As soon as the signal sequence is produced by the ribosome, the signal-recognition-particle (SRP) recognises the sequence and binds to the sequence. The SRP transports the ribosome with the polypeptide chain to the ER, where the SRP binds to a receptor and by hydrolysing GTP, a protein channel opens. This allows the SRP to dissociate and bind to another signal sequence of a different polypeptide chain. The ribosome continues to translate the mRNA, with the polypeptide chain being fed into the ER lumen. An enzyme, signal peptidase, removes the signal sequence from the polypeptide chain and the growing amino acid sequence enters the lumen of the ER. Once the translation is completed, the completed polypeptide chain remains in the ER to be modified while the ribosome returns to the cytosol to translate further mRNA chains.
For those proteins destined for the plasma membrane, the signal sequence or another region of hydrophobic amino acids will cause the protein to be embedded in the ER membrane and anchors the protein in the membrane to hold it there.
Proteins Targeted To Lysosomes
Proteins destined for lysosomes will be modified in the Golgi by the addition of a mannose-6-phosphate group to mannose residues on N-linked oligosaccharides, and this process indirectly requires ATP. Proteins only receive the addition of the mannose-6-phosphate group if they contain a signal patch; this is a series of specific amino acids from various parts of polypeptide chain that give the protein a specific three-dimensional folded structure.
The mannose-6-phosphate groups are recognised by a mannose-6-phosphate receptor on the trans Golgi apparatus and leads to the protein being packaged into a vesicle, which then pinches off and is transported to lysosomes. When the vesicle containing the lysosomal protein reaches the lysosome, the acidic environment of the lysosome causes the protein to disassociate from the receptor, and the receptor travels back to the Golgi apparatus to reused. To prevent the lysosomal protein from returning to the Golgi apparatus, the phosphate group is removed and this allows the
protein to carry out its function in the lysosome.
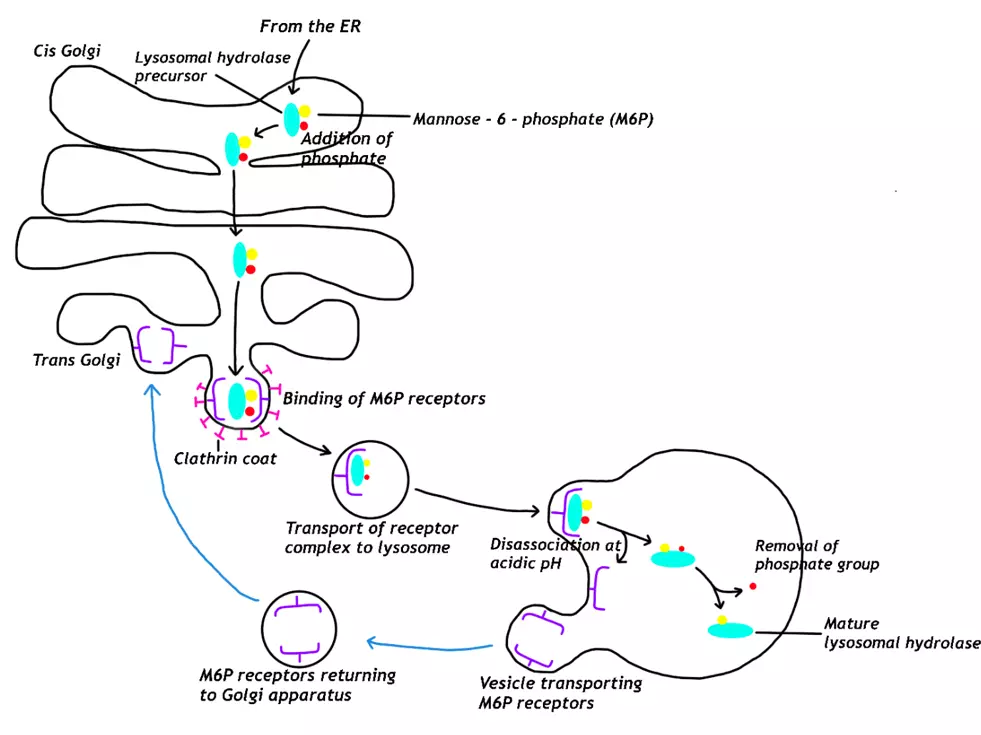
Diagram - The pathway for proteins targeted for lysosomes from the golgi apparatus
SimpleMed original by Dr. Peter Parkinson
Proteins That Remain in the Endoplasmic Reticulum
Some proteins have a function that is involved with the mechanisms and processes that take place in the ER. These proteins will contain a signal sequence at the C-terminal of the polypeptide chain. This signal is a sequence of four amino acids, which is Lysine-Aspartate-Glutamate-Leucine (KDEL).
If a folded protein containing the KDEL signal is transported to the cis-Golgi apparatus via a vesicle, the KDEL signal will bind to a KDEL receptor in the Golgi and this process is enhanced by the low pH environment present. The ER protein-KDEL receptor complex is transported back to the ER and due to the neutral pH environment of the ER, the protein dissociates from the receptors and the KDEL receptor returns to the Golgi apparatus.
Proteins Targeted to the Plasma Membrane and Secretion
For proteins destined for the plasma membrane or secretion out of the cell, there is no specific signal sequence that is detected during translation at the ribosome or during modification in the ER or Golgi apparatus. This leads to these proteins being packaged in a vesicle that is taken to the plasma membrane to become part of the plasma membrane or be secreted into the exterior environment via exocytosis.
Edited by: Dr. Thomas Burnell
Reviewed by: Dr. Marcus Judge
- 13977

