Next Lesson - Membrane Potentials
Abstract
- Cell membranes are semi-permeable, allowing some substances to cross but not others.
- Whether a substance moves by passive or active transport depends on whether there is a favourable electrochemical gradient.
- Simple diffusion is the passive movement of molecules across the phospholipid bilayer.
- Facilitated diffusion is the passive movement of molecules/ions via channel proteins.
- Active transport is the movement of molecules/ions against an unfavourable gradient, requiring energy from ATP hydrolysis.
- Cellular pH is controlled by the movement of hydrogen and bicarbonate ions.
- Cell volume is controlled by the movement of osmotically active ions.
- Calcium is an important signalling ion and is carefully regulated by the cell via a variety of transporters and channel proteins.
Core
Cellular membranes are described as semi-permeable as they allow some substances to pass through but not others. The cell membrane is composed of a phospholipid bilayer and membrane proteins (for more info check out our article on Biological Membranes). The permeability of a molecule therefore depends on its ability to pass through either of these components.
The phospholipid bilayer allows both hydrophobic and small, polar (uncharged) molecules to freely diffuse through it. Examples include O2, CO2, water, urea, and glycerol. The lipid bilayer is impermeable to charged ions and large polar molecules such as glucose.
The membrane’s permeability to ions and larger molecules, however, is dependent on the expression of protein channels and transporters. This can vary depending on cell type - for example, neurones and muscle cells have a much higher permeability to Na+ and K+ compared to non-excitable cells due to a higher frequency of Na+ and K+ channels involved in generating the action potential.
Passive transport is via diffusion, which occurs down an electrical or chemical (i.e. concentration) gradient without requiring energy input. Diffusion through the lipid bilayer itself is known as simple diffusion, whereas diffusion via a channel protein is facilitated diffusion. Unlike simple diffusion, facilitated diffusion is a saturable process (i.e. it will reach a maximum rate) due to a limited number of available channels in the membrane.
Many ion channels are only open under certain circumstances and are said to be ‘gated’. There are 3 main types of gated ion channel:
- Ligand-gated (e.g. nicotinic acetylcholine receptor) - These channels open or close when a chemical signalling molecule (ligand) binds to it.
- Voltage-gated (e.g. voltage-gated calcium channel) - These channels open in response to changes in membrane potential (typically depolarisation).
- Mechanically-gated (e.g. mechanoreceptors of the skin) - These channels open in response to mechanical stretch of the membrane.
To determine whether a substance moves via passive or active transport, we must determine whether there is a favourable electrochemical gradient for diffusion. If there is a gradient, the substance will move passively, however if there is not it will require active transport. To quantify this, we can calculate the free energy change (ΔG). A negative ΔG indicates passive diffusion whereas a positive ΔG indicates active transport.
For uncharged molecules, we are only concerned with the concentration gradient. We use the formula: ΔG = RT. loge([X]in/[X]out); where R is the gas constant (8.314 J mol-1 K-1), T is temperature (K), and [X]in and [X]out are the concentrations on the inner and outer sides of the membrane respectively.
For ions, we must also take into account the electrical gradient (i.e. membrane potential) - positively charged ions will tend to move to areas of negative charge and vice versa. We use the formula: ΔG = RT. loge([X]in/[X]out) + ZF. ΔΨ; where RT. loge([X]in/[X]out) is the same as above, Z is the charge on the ion (e.g. sodium is +1), F is the Faraday constant (96485 C mol-1) and ΔΨ is the membrane potential (V).
Active transport is the movement of ions/molecules against an unfavourable electrochemical gradient, requiring energy from ATP hydrolysis. Active transport is a very energy-consuming process and can account for a large proportion of a cell’s basal metabolic rate, particularly in excitable tissues.
Primary active transport is where the energy is derived directly from ATP hydrolysis. This is achieved by pump proteins, or ATPases: examples include the Na+/K+ ATPase (also see article on Membrane Potential) and the plasma membrane Ca2+ ATPase (PMCA).
Secondary active transport, otherwise known as co-transport, utilises the energy from a pre-existing electrochemical gradient, typically Na+; the pre-existing Na+ gradient is generally set up by primary active transport, such as the Na+/K+ ATPase. Co-transport systems can be further classified into symports and antiports.
- Symports, such as Na+-glucose co-transport (e.g. SGLT1), are where the ions/molecules flow through the transporter in the same direction.
- Antiports, such as the Na+/H+ exchanger, are where the ions/molecules flow in opposite directions across the membrane.
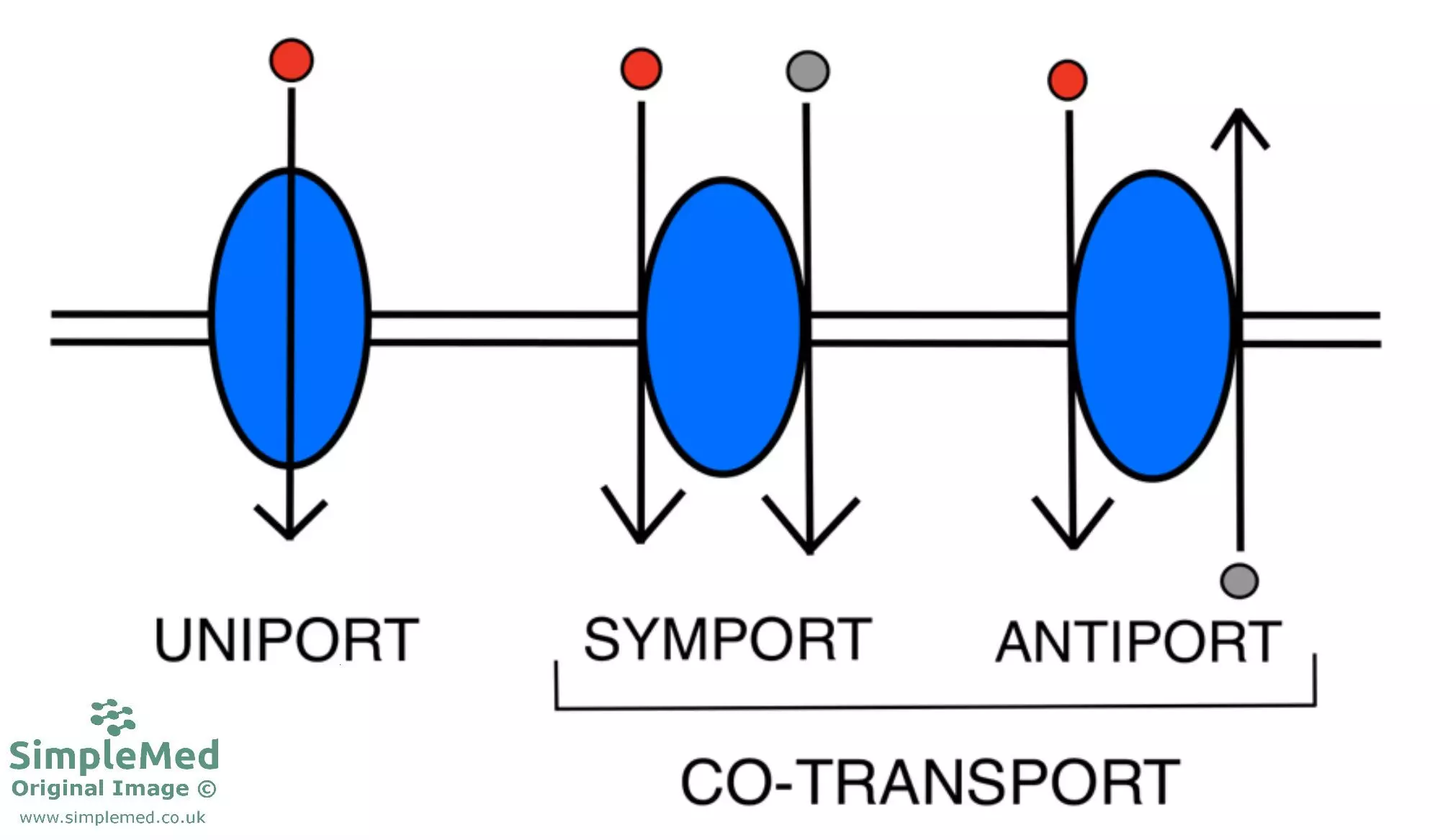
Diagram - The classification of the different types of cell membrane transporters
SimpleMed original by Dr. Joshua Bray
Cellular pH and Volume Regulation
Intracellular pH is regulated by the movement of H+ (acid) and HCO3- (base) into or out of the cell. This can be achieved by a variety of transporter proteins on the cell surface membrane.
The Na+/H+ exchanger is an acid extruder and therefore alkalinises the cell (raises pH). It uses a pre-existing Na+ gradient to move 1 Na+ into and 1 H+ out of the cell (secondary active transport). This transporter is found on most cells, but is particularly important in the kidney tubule, where it can be targeted by amiloride (a K+ sparing diuretic).
The Na+/HCO3- co-transporter causes alkali influx and therefore also alkalinises the cell. It uses a pre-existing Na+ gradient to move Na+ together with HCO3- into the cell (the exact stoichiometry depends on the transporter isoform and tissue). This transporter is found on a wide variety of cells.
The anion exchanger (AE), or Band 3, is an alkali extruder and therefore acidifies the cell. It moves 1 Cl- in and 1 HCO3- out. This transporter is found on erythrocytes and also in the kidney tubule.
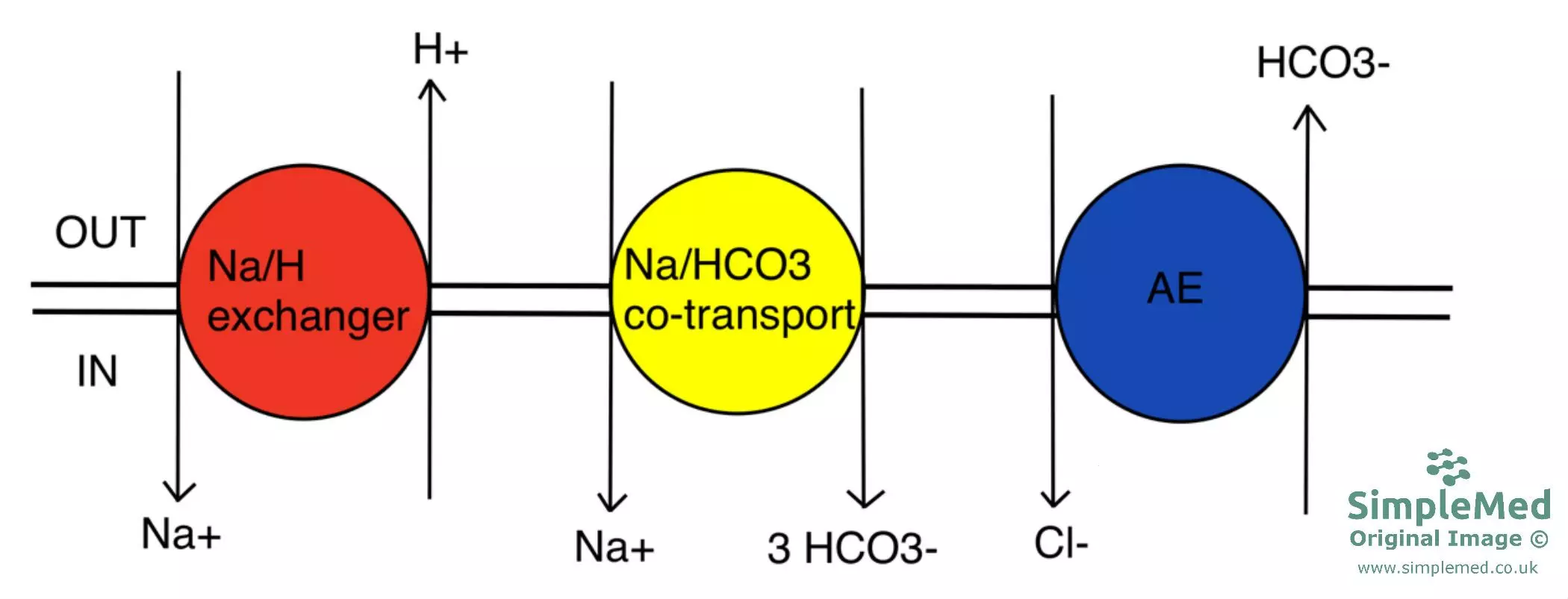
Diagram - The channels involved in cellular pH regulation and the directions the ions travel
SimpleMed original by Dr. Joshua Bray
The principle of cell volume regulation is the movement of osmotically active ions (e.g. Na+, K+, Cl-) into or out of the cell, so that water follows by osmosis. Influx of ions will cause the cell volume to increase whereas efflux of ions will cause the cell volume to decrease.
Intracellular Calcium Regulation
Calcium, Ca2+, is an important ion involved in a variety of intracellular signalling pathways. Calcium regulates a wide range of physiological and pathological processes: muscle contraction, neurotransmission, fertilisation, metabolic regulation, cell death (apoptosis/necrosis), learning and memory.
The human body contains over 1kg of calcium, however more than 99% of this is locked away in bone as insoluble hydroxyapatite [Ca5(PO4)3(OH)]. The remaining 1% is distributed throughout the extracellular and intracellular fluid. Most of the body’s soluble calcium is found in the extracellular compartment (i.e. the blood and interstitial fluid), although approximately half of this is bound to plasma proteins such as albumin. The concentration of Ca2+ inside the cell’s cytoplasm is actually very low and kept within tight limits, as high concentrations of Ca2+ are directly toxic to the cell. The cell does however have a store of Ca2+ within the endoplasmic reticulum (or sarcoplasmic reticulum for muscle cells).
Normal Ca2+ concentrations are as follows:
- Extracellular [Ca2+] = 1-2 x 10-3 M
- Cytosolic [Ca2+] = 1 x 10-7 M (= 100 nM)
- ER/SR [Ca2+] = 2-3 x 10-4 M
The resting concentration of Ca2+ inside the cell is controlled by multiple protein transporters:
- Na+/Ca2+ Exchanger (NCX) - This transporter is found on the plasma membrane and normally moves 3 Na+ into and 1 Ca2+ out of the cell. It has a low affinity but high capacity, therefore it is responsible for the bulk of Ca2+ extrusion.
- Plasma Membrane Ca2+ ATPase (PMCA) - This is a Ca2+ pump found on the plasma membrane which moves Ca2+ out of the cell using ATP hydrolysis. This transporter has a high affinity but low capacity, therefore it is responsible for the ‘fine-tuning’ of intracellular calcium levels.
- Sarco(endo)plasmic Reticulum Ca2+ ATPase (SERCA) - This is the equivalent of PMCA but on the sarco-/endoplasmic reticulum. It is responsible for replenishing Ca2+ stores in the SR/ER.
Many cell signalling pathways involve a rise in intracellular calcium. This increase can be achieved using a variety of different Ca2+ channels:
- Voltage-gated Calcium Channel (VGCC) - This channel protein causes Ca2+ influx in response to depolarisation of the plasma membrane.
- IP3 Receptor (IP3R) - This receptor is a ligand-gated ion channel and is found on the SR/ER. Its ligand is IP3, which is generated by the activation of GPCRs coupled to G-alphaq, which in turn activates phospholipase C. IP3 binding results in opening of the channel and movement of Ca2+ from the SR/ER into the cytoplasm.
- Ryanodine receptor (RyR) - This receptor is another ligand-gated ion channel found on the SR/ER. This receptor is involved in a process known as calcium-induced calcium release (CICR), where the binding of Ca2+ to RyRs results in the opening of the RyRs and an increased Ca2+ efflux from the SR/ER.
- This process serves to amplify small increases in intracellular Ca2+ concentration; during increases in intracellular Ca2+, the majority of these extra Ca2+ ions are derived from CICR.
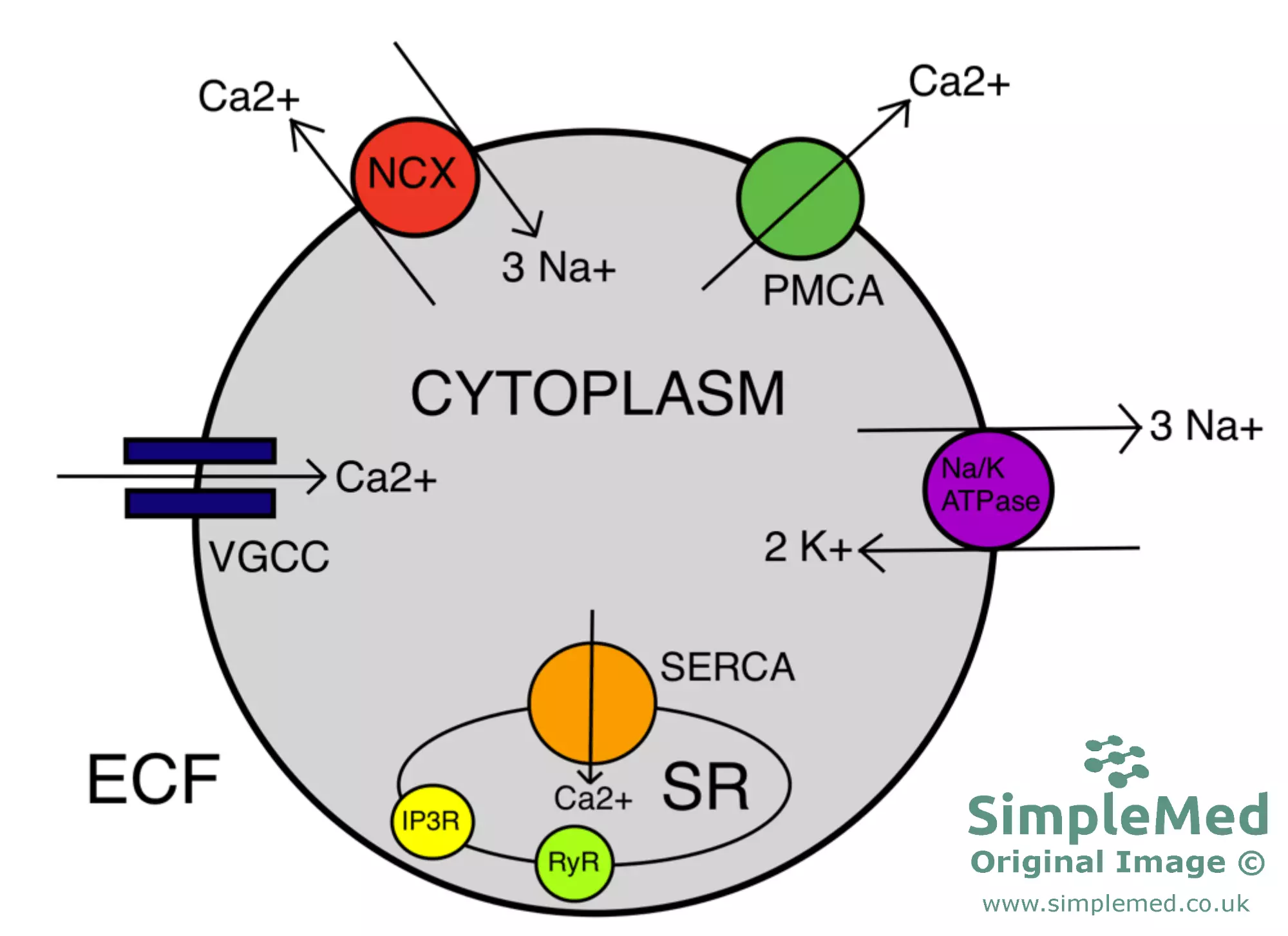
Diagram - A summary of cellular calcium ion regulation
SimpleMed original by Dr. Joshua Bray
Reviewed by: Dr. Marcus Judge
- 15209

