Next Lesson - Alcohol Metabolism
Abstract
- Nitrogen is an important molecule in the body, used in DNA/RNA and neurotransmitter synthesis. The relationship between nitrogen entering the body and nitrogen leaving the body is called the nitrogen balance.
- Amino acids can have many functions, and are made up of three classes: glucogenic (can form glucose), ketogenic (can form ketone bodies) or both. To be used as a source of energy, amino acids must undergo either transamination or deamination to create a usable carbon skeleton, which can then be used in respiration.
- Urea is an important waste molecule in the body, and is produced from protein metabolism; it relates to refeeding syndrome.
- Homocystinuria and phenylketonuria are important diseases relating to defects in amino acid metabolism.
Core
Nitrogen is an important molecule within the body. It is used in DNA/RNA, neurotransmitters, and hormones. Therefore humans need a constant supply. The main source of nitrogen is supplied by protein in the diet as they are broken down into amino acids and then absorbed at the intestinal wall.
Nitrogen in the body is referred to as ‘balanced’, which defines the relationship between nitrogen entering the body (through diet) and nitrogen leaving the body (through loss of skin and waste).
Nitrogen balance can be:
- In equilibrium, intake = loss. This is a normal nitrogen balance.
- In a positive nitrogen balance: intake > loss. This occurs in growth and pregnancy as more protein is required for growth.
- In a negative nitrogen balance: intake < loss. This occurs with trauma, malnutrition, or infection, and can also be seen during short-term fasting or with ageing.
In addition to being used in protein synthesis, amino acids can have special functions of their own. For example, glycine and glutamate are both amino acids and important neurotransmitters.
- Tyrosine - production of noradrenaline and melanin which is the pigment formed by melanocytes in the skin.
- Cysteine - glutathione production, which is important for “mopping up” any free radical species that could go on to cause cell damage.
- Tryptophan - producing serotonin, which is an important neurotransmitter in the brain.
- Histidine - producing histamine, an important chemical involved in the inflammatory process.
- Glutamate - production of GABA, which is an inhibitory neurotransmitter in the brain.
- Glycine - production of haem and collagen used to make haemoglobin, and purines used in DNA/RNA replication.
- Glucogenic amino acids can be converted to glucose through gluconeogenesis, e.g. Alanine, Aspartate.
- Ketogenic amino acids can be converted into Acetyl CoA (a precursor of ketone bodies that can be used in respiration), e.g. Lysine, Leucine.
- Amino acids can also be both ketogenic and glucogenic, meaning they can be converted to glucose or ketone bodies, e.g. Threonine, Tyrosine, Tryptophan.
- Glucogenic begins with A - Alanine and Aspartate
- Ketogenic begins with L - Lysine and Leucine
- Those in both classes begin with T - Threonine, Tyrosine, Tryptophan
Unfortunately, not all amino acids fit this pattern, such as phenylalanine which is ketogenic (important, see later).
- Insulin ("the hormone of plenty") increases protein synthesis and decreases amino acid release. This is because high concentrations of insulin indicate that there is lots of glucose available in the blood, so no gluconeogenesis is needed. Growth hormone also exhibits similar effects to insulin.
- Glucocorticoids (like cortisol) increase amino acid release for gluconeogenesis and decrease protein synthesis. This is because high levels of cortisol indicate that more glucose is needed in the blood. This relates to Cushing’s Syndrome, where excess cortisol causes excessive protein breakdown, causing characteristic thin skin and purple abdominal striae.
To be used in the production of energy, amino acids have to have their amino group removed so that only carbon skeletons remain. This can be done in two ways:
Transamination is the process that swaps the amino group (NH2) on an amino acid for a carboxyl group (C=O). This can be done through two enzymes called alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Both of these reactions are reversible, meaning they can be used to produce amino acids (alanine or aspartate from glutamate) or substrates for energy production (pyruvate or oxaloacetate).
- ALT catalyses the movement of the amino group from alanine to alpha-ketoglutarate, producing pyruvate and glutamate.
- AST catalyses the movement of the amino group from aspartate to alpha-ketoglutarate, producing oxaloacetate and glutamate.
The second method of producing energy from amino acid metabolism is through deamination. This involves the liberation of the amino group as free ammonia, which is eventually turned into urea through the urea cycle.
Urea is a molecule in the body with a high nitrogen content that is chemically inert in humans (meaning it doesn’t react with anything). It is produced primarily following deamination of glutamate in the liver which releases ammonia. As ammonia is toxic to human cells, it is converted to urea which is a safer (water soluble) waste product which is released in the urine.
Ammonia is converted to urea during the urea cycle, a process involving 5 liver enzymes. The urea cycle consists of four enzymatic reactions: one mitochondrial and three cytosolic.
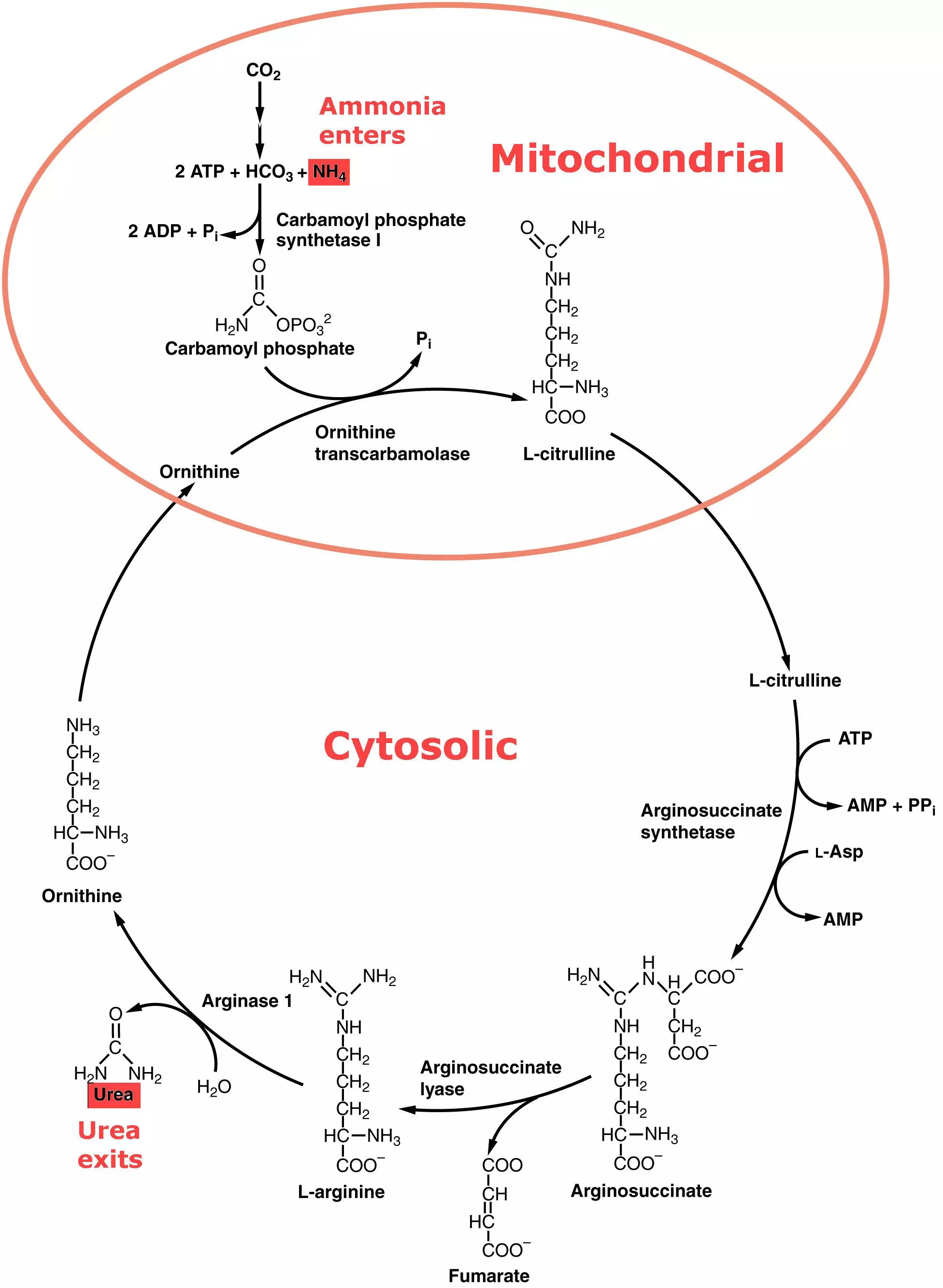
Diagram - The Urea Cycle with enzymes and products. A knowledge of the enzymes is not necessary, but understanding that they can be up/down-regulated and how this might affect the production of Urea from Ammonia is
Creative commons source OpenStax College, edited by Dr. Marcus Judge [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)]
Having a high protein diet (meaning there are a lot of amino acids to metabolise), means that the enzymes for the urea cycle are upregulated, so the increased amount of protein can be metabolised faster and so no ammonia builds up.
Having a very low protein diet such as in starvation can lead to down-regulation of these enzymes because they are not needed to metabolise protein.
Having a low protein diet for extended periods of time is dangerous as it can contribute to Refeeding Syndrome when nutrition is reintroduced after a period of malnutrition, particularly if this is rapid.
This leads to the massive attempted synthesis of glycogen, fat and protein in cells and causes largely deranged levels of potassium, magnesium and phosphorus in the blood. Although refeeding syndrome has multiple contributors, ammonia build up also plays into its pathogenesis. When protein intake is low, the enzymes of the urea cycle are downregulated, meaning that when protein is consumed again the enzymes are overwhelmed and cannot convert ammonia into urea fast enough causing ammonia to build up (hyperammonaemia). High ammonia is harmful because it interferes with the Krebs Cycle, the blood-brain barrier, protein synthesis and pH. Ammonia is basic, meaning it can also affect local pH.
Cardiac, pulmonary and neurological symptoms can occur due to refeeding syndrome and the low serum levels of potassium, magnesium and phosphorus, if severe enough, can be fatal.
Similar symptoms and pathophysiologies can also happen in genetic deficiencies of the enzymes of the urea cycle, but these can be managed with a low protein diet.
There are many other disorders that occur due to inadequate levels of enzymes needed to metabolise amino acids.
Phenylketonuria (PKU) is a deficiency in phenylalanine hydroxylase meaning phenylalanine cannot be converted to tyrosine. This causes phenylalanine to build up in the blood, and with transamination, so do phenylketones (due to diversion into alternative metabolic pathways). Symptoms include developmental delay, small head, seizures and hypopigmentation. PKU along with other inborn errors of metabolism are tested for in the blood test all newborn babies must undergo, called the heel prick test. This means it is often caught and treated early in the UK. The management for phenylketonuria is a low phenylalanine, high tyrosine diet.
Homocystinuria is another syndrome due to enzymatic deficiency. Homocysteine (with an ‘e’) is made from methionine and is normally broken down by cystathionine beta-synthase. In Homocystinuria this enzyme is deficient, meaning that methionine cannot properly be broken down, so homocysteine (with an ‘e’) builds up in the blood, and is oxidised to homocystine (without an ‘e’ - this is two homocysteine molecules bound together). This homocystine (without an ‘e’) is excreted in the urine. The symptoms of homocystinuria can sometimes be confused with Marfan’s Syndrome, because it presents with the similar symptoms of lens dislocation, skeletal deformities, and defective connective tissue. However, Marfan’s Syndrome would not present with high blood levels of homocystine. Homocystinuria is also assessed for in the heel prick tests in newborns.
- Cysteine - an amino acid, unrelated to homocystinuria
- Cystine - 2 x cysteine bound together with a disulphide bond, also unrelated
- Homocysteine - made from methionine
- Homocystine - 2 x homocysteine (oxidised form)
Reviewed by: Dr. Marcus Judge
- 9663

