Next Lesson - Lipid Metabolism and Transport
Abstract
- The oxygen-dependent pathways follow from glycolysis. They include the Link reaction, the Krebs cycle and Oxidative Phosphorylation.
- The Link reaction turns pyruvate into Acetyl CoA, which is joined with oxaloacetate in the Krebs cycle, forming citrate, which is cycled around, producing a number of intermediates and co-factors.
- The electron transport chain (ETC) is the site at which oxidation of NADH and FADH2 occurs to set up a proton motive force (PMF). This PMF is set up by protons transported across the mitochondrial inner membrane and then is released through ATP synthase to produce ATP.
- Oxidative phosphorylation (or the ETC) is oxygen dependent, because oxygen acts as the terminal electron acceptor.
Core
The oxygen-dependent pathways ultimately rely on a constant supply of oxygen because reducing equivalents generated earlier are re-oxidised at the mitochondrial membrane to produce ATP. Without oxygen, oxidative phosphorylation cannot proceed and these pathways slow or stop.
These processes occur after the completion of Glycolysis, of which the final product is pyruvate. They include the Link reaction, the Krebs cycle and Oxidative Phosphorylation.

Diagram - The Link Reaction. Note the modulatory enzyme pyruvate dehydrogenase
SimpleMed original by Dr. Maddie Swannack
The Link reaction, seen above, ‘links’ the end product of Glycolysis and the Krebs Cycle, and is irreversible due to the loss of carbon dioxide.
Pyruvate dehydrogenase is the multi-enzyme complex required for this reaction. It requires many co-factors to work effectively, all supplied by B vitamins. A deficiency in this enzyme or any of its co-factors can lead to lactic acidosis, as pyruvate cannot be turned into Acetyl CoA and the anaerobic pathway must work to produce energy, leading to lactic acid build up.
Pyruvate dehydrogenase is allosterically regulated by a number of compounds:
- It is activated by high concentrations of: pyruvate, NAD+, ADP and insulin - all present when there is low energy stores in the body (or excess glucose).
- It is inhibited by high concentrations of: Acetyl CoA, NADH, ATP, and citrate - all present when there is high energy stores in the body (or insufficient glucose).
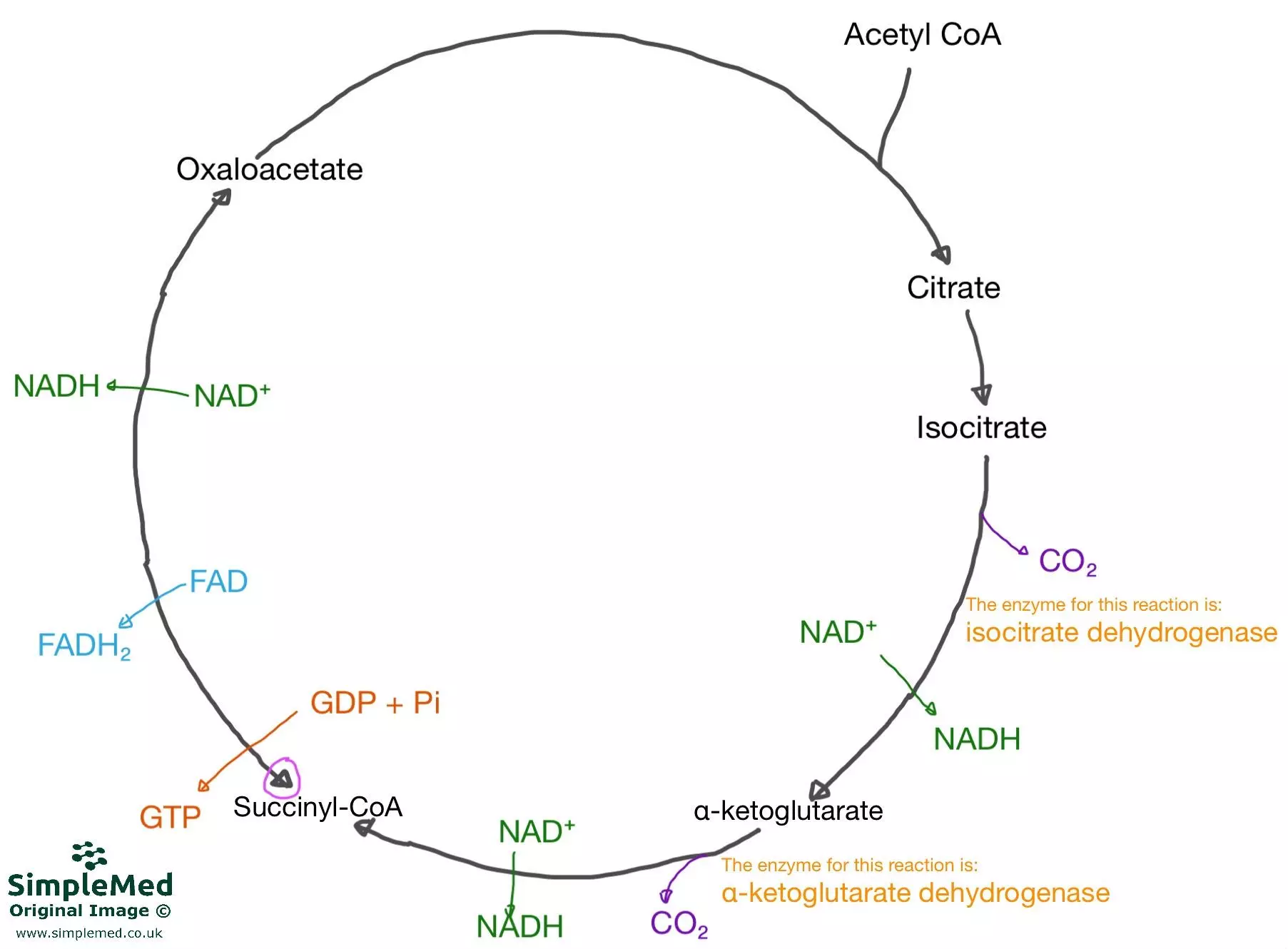
Diagram - The Krebs cycle, showing the basic intermediates, the products of each reaction and the key enzymes involved. Acetyl CoA from the Link Reaction enters and is combined with oxaloacetate to produce citrate
SimpleMed original by Dr. Maddie Swannack
Per glucose molecule (2 cycles of the Krebs Cycle), the Krebs Cycle produces:
- 6x NADH
- 2x FADH2
- 2x GTP
The pink-circle-highlighted-arrow visible on the diagram of the Krebs Cycle below highlights that the reaction between the C4 intermediate and oxaloacetate is reversible, because there is no loss of carbon dioxide in this step, however, it would be rare for the reaction to reverse.
Therefore, so far in the metabolism of glucose:
- All C-C bonds have been broken and oxidised to CO2
- All C-H bonds have been broken and transferred H+ and e- to NAD+/FAD
The FADH2 and NADH produced in the Krebs cycle (as well as the NADH produced by Glycolysis) provide reducing power for oxidative phosphorylation, which occurs at the mitochondrial membrane.
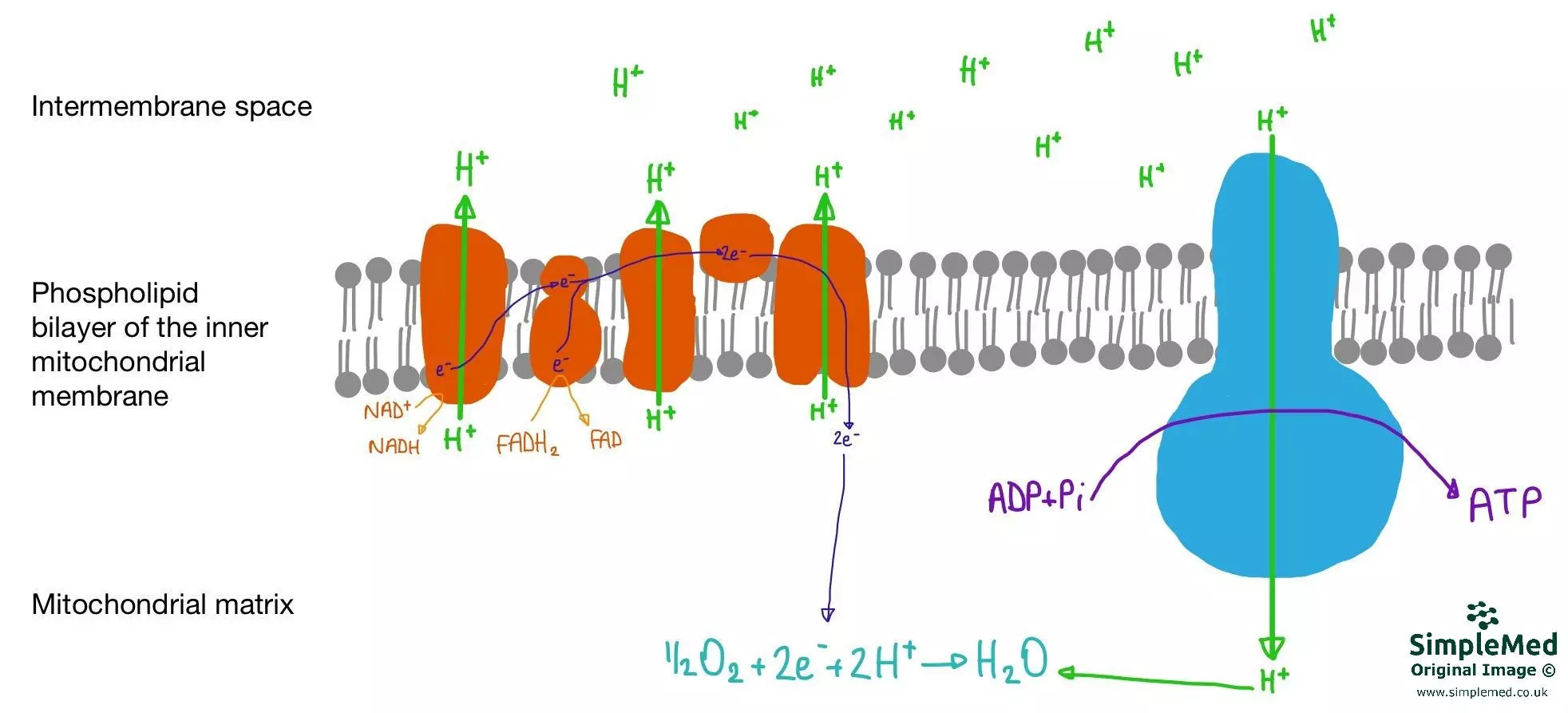
Diagram - The process of Oxidative Phosphorylation occurring at the mitochondrial membrane. Note the blue channel, ATP synthase, through which the H+ ions travel back down the proton motive force to convert ADP to ATP
SimpleMed original by Dr. Maddie Swannack
NADH and FADH2 from Glycolysis and the Krebs Cycle are re-oxidised with O2 at the mitochondrial membrane to create energy. The electrons from the NADH and FADH2 are donated to the electron transport chain within the mitochondrial membrane. As these electrons travel down the energy levels through the electron transport chain, they cause the release of energy. At the end of the chain, they combine with free oxygen (known as the terminal electron acceptor) to form water.
The energy from this oxidation reaction is used to power proton pumps (Proton Translation Complexes) that move H+ from the matrix of the mitochondria to the intermembrane space. This creates a potential difference of approximately -180mV across the membrane. This H+ concentration gradient is called the proton motive force.
The potential difference created causes an attractive force for the H+ - under normal coupled conditions, their main path back into the mitochondrial matrix is via the ATP synthase enzyme (blue on the diagram above). Their movement back through this enzyme channel leads to the phosphorylation of ADP to ATP, as seen below.
![]()
Diagram - The conversion of ADP to ATP by ATP synthase within the mitochondrial membrane wall
SimpleMed original by Dr. Maddie Swannack
This normally occurs in the direction of ATP synthesis, with ATP synthase powered by the H⁺ gradient.
- 2 moles of NADH produces 5 moles of ATP
- 2 moles of FADH2 produces 3 moles of ATP
Inhibitors can block electron transport by blocking the action of oxygen as the terminal electron acceptor, meaning that no proton gradient can be established. An example of an ETC inhibitor is cyanide; the action of inhibiting the ETC is what leads to its lethality.
Reviewed by: Dr. Marcus Judge
- 9146

