Next Lesson - Autonomic Nervous System Introduction
Abstract
- The action potential is the basis of electrical communication in excitable cells such as neurones.
- The neuronal action potential relies on the movement of Na+ and K+ ions across the cell membrane.
- The action potential is predictable and ‘all or nothing’ in nature.
- The refractory period describes the period of time where the cell cannot generate another action potential.
- Local anaesthetics work by blocking Na+ channels.
- The action potential is propagated via the local spread of current which triggers new action potential generation.
- Conduction velocity in neurones is substantially increased via myelination.
- Neurones interact with skeletal muscle cells at the neuromuscular junction.
- Drugs which block the neuromuscular junction can be used as muscle relaxants during general anaesthesia.
- Myasthenia gravis is an autoimmune condition affecting transmission at the neuromuscular junction.
Core
The action potential describes the phenomenon by which excitable cells create an electrical signal via the movement of ions across the membrane. The key features of an action potential are:
- It relies on ionic gradients - Pre-existing ionic gradients are required for the movement of ions across the membrane. Changing the membrane’s permeability to different ions (i.e. opening and closing ion channels) allows the cell’s membrane potential to be changed.
- It is predictable in nature - Although the shape of the action potential can vary between excitable cell types, in a particular cell type (e.g. a neurone) the action potential should be the same every time.
- It is ‘all or nothing’ - For an action potential to be generated, the voltage across the membrane must reach a threshold level; any lower than this threshold and no action potential will be fired.
- It is propagated without loss of amplitude - The strength of the action potential is maintained along the length of the axon as the local spread of depolarisation triggers new action potentials to be generated.
The action potential relies on the movement of Na+ and K+ ions. Recall that Na+ influx causes depolarisation, whereas K+ efflux causes hyperpolarisation.
The stages of the action potential are as follows:
- Initial stimulus - This is the initial depolarisation that triggers the action potential; it is generally due to the movement of Na+, either due to the activation of receptors or the local spread of depolarisation from an adjacent action potential.
- Depolarisation - If the initial depolarisation reaches the threshold level, around -55 mV, voltage-gated Na+ channels (VGSCs) open which results in rapid depolarisation.
- Repolarisation - After the membrane is fully depolarised, the membrane becomes more negative again as VGSCs become inactivated and voltage-gated K+ channels open.
- Hyperpolarisation - Often the cell ‘overshoots’ the repolarisation phase due to the movement of K+, resulting in a brief period of hyperpolarisation before returning back to the resting membrane potential.
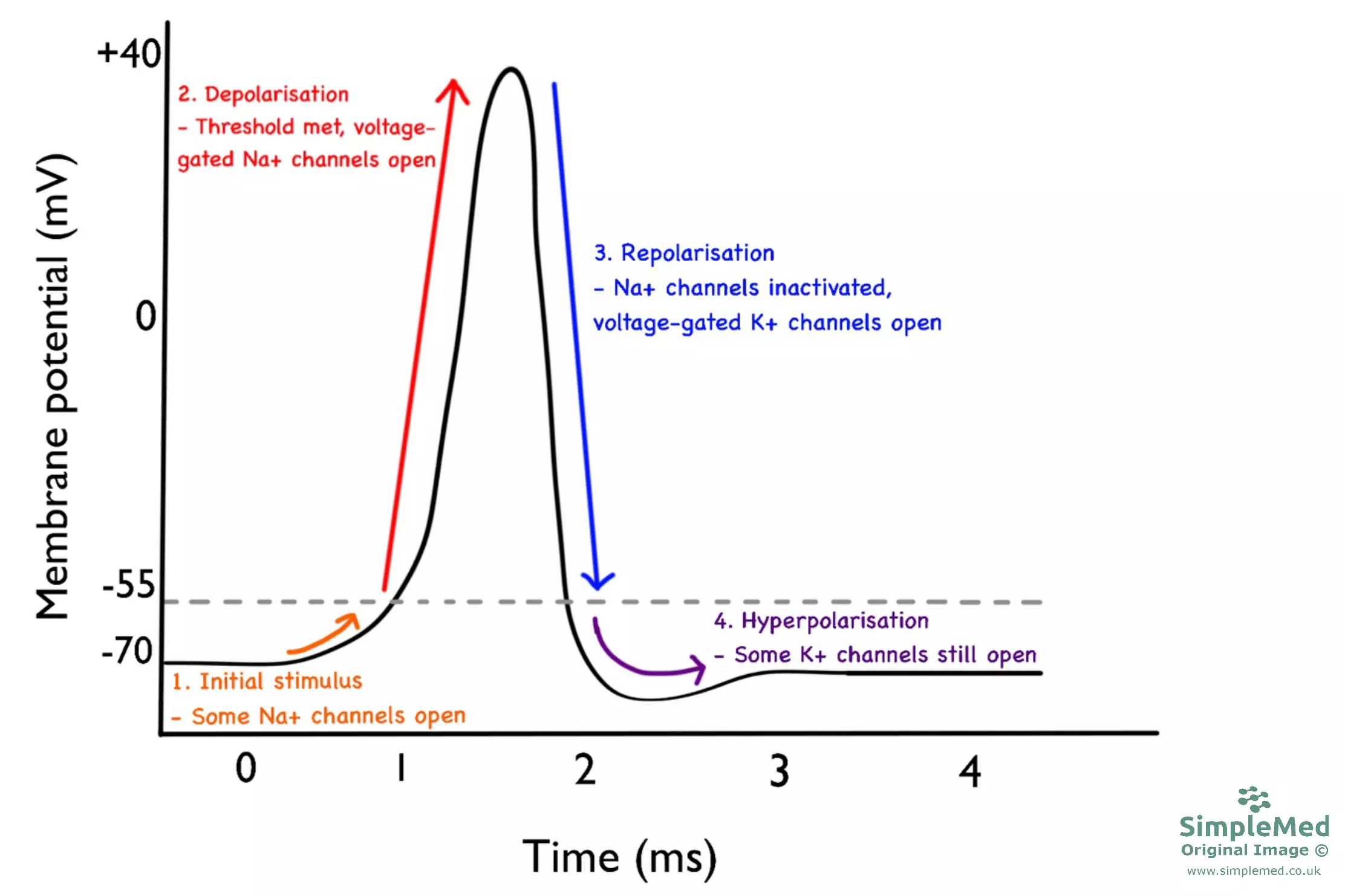
Diagram - Graph showing the stages of the neuronal action potential
SimpleMed original by Dr. Joshua Bray
Voltage-Gated Na+ Channels and the Refractory Period
The voltage-gated Na+ channel is different from other ion channels in that apart from being open or closed, it also has an ‘inactivated’ state. VGSCs become inactivated in response to depolarisation and in this state Na+ ions cannot pass through the channel. From the inactivated state, the channel must first ‘recover’ into the closed state before it can be open again. This provides the basis for the refractory period.
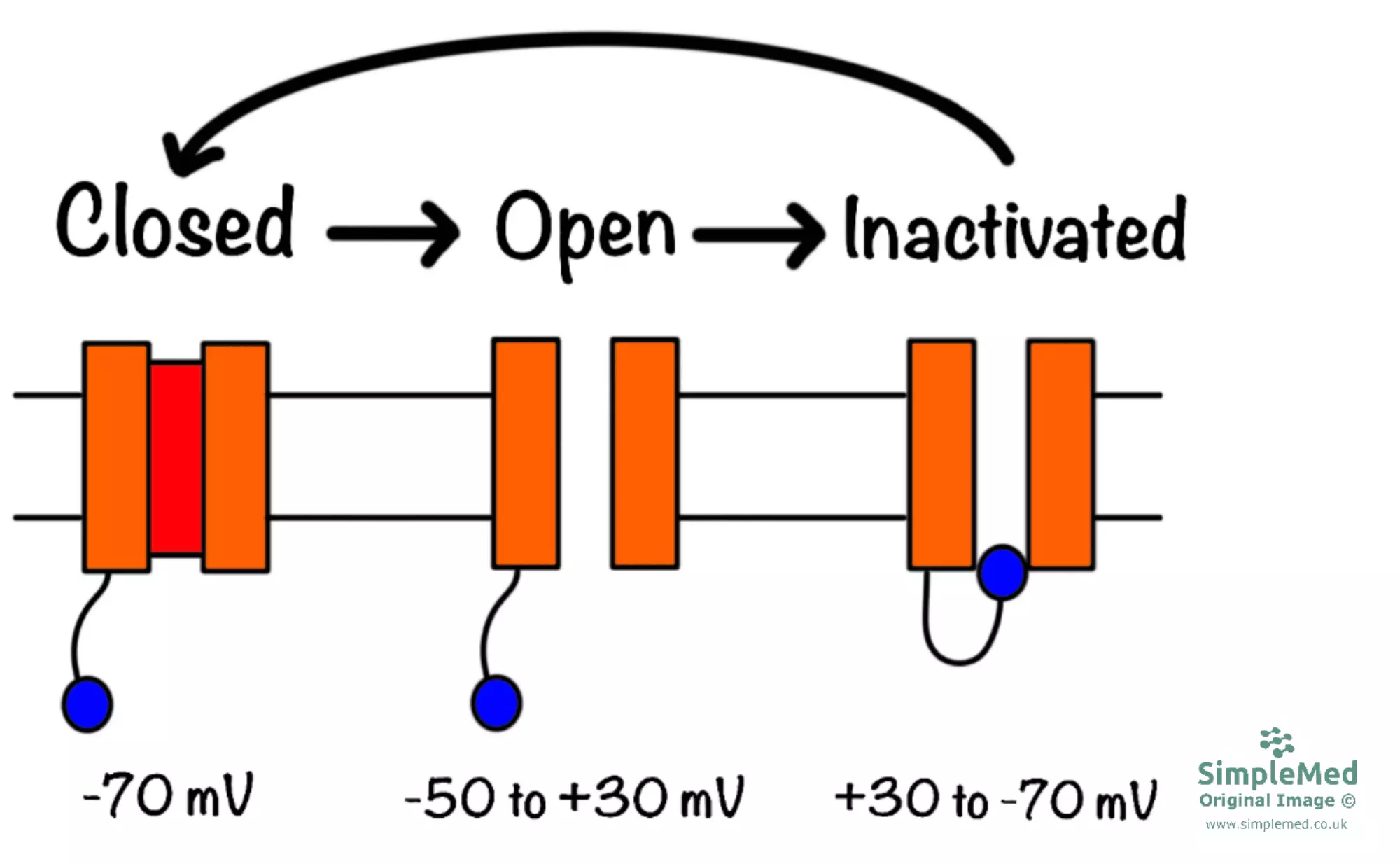
Diagram - Voltage-gated Na+ channels in the closed, open and inactivated states
SimpleMed original by Dr. Joshua Bray
The refractory period describes the period of time in which the cell cannot generate an action potential. There are 2 terms that you should be familiar with:
- Absolute Refractory Period - Within this period, the cell cannot generate an action potential whatsoever, as all Na+ channels are in the inactivated state.
- Relative Refractory Period - In this period, the cell can generate another action potential, although it is harder to do so. This is because the VGSCs are beginning to recover, although some are still inactive. The relative refractory period ends when all Na+ channels have recovered.
Local anaesthetics, such as lidocaine, work by blocking Na+ channels in small afferent neurones responsible for pain. By blocking these channels, it prevents depolarisation so an action potential cannot be generated. It is often referred to as a ‘use-dependent’ block, meaning that the drug has a preference for blocking Na+ channels which are in the open or inactivated state.
Propagation of the Action Potential
It is necessary for the action potential to travel, or be propagated, along the entire length of the axon (which can be up to 1 meter long!). To do this, there is a local spread of current which triggers new action potentials to be generated along the length of the axon.
Local current theory dictates that depolarisation at a given point in the membrane causes a spread of depolarisation in the surrounding area of membrane. This is because an influx of Na+ ions repels other positive ions in the cell, resulting in local depolarisation. However, the further this current spreads, the weaker it gets.
The length constant (λ) is the distance travelled before the membrane potential falls to 37% of its original value (i.e. the higher the length constant, the further the spread of charge without loss of amplitude). The length constant is determined by 2 factors:
- Membrane Capacitance (Cm) - This is the ability to store charge. The higher the capacitance, the slower the change in membrane potential (lower length constant).
- Membrane Resistance (Rm) - This is determined by the number of open ion channels. The fewer channels open, the higher the resistance and the further a change in voltage will spread along the axon (higher length constant).
Nervous transmission is made quicker by myelination of nerve fibres. Axons are wrapped in a fatty substance called myelin, but small portions of axon are left unmyelinated and these are termed Nodes of Ranvier. It is very important to note that a myelinated axon has very few ion channels along the internodal membrane, with channels being highly concentrated at the Nodes of Ranvier.
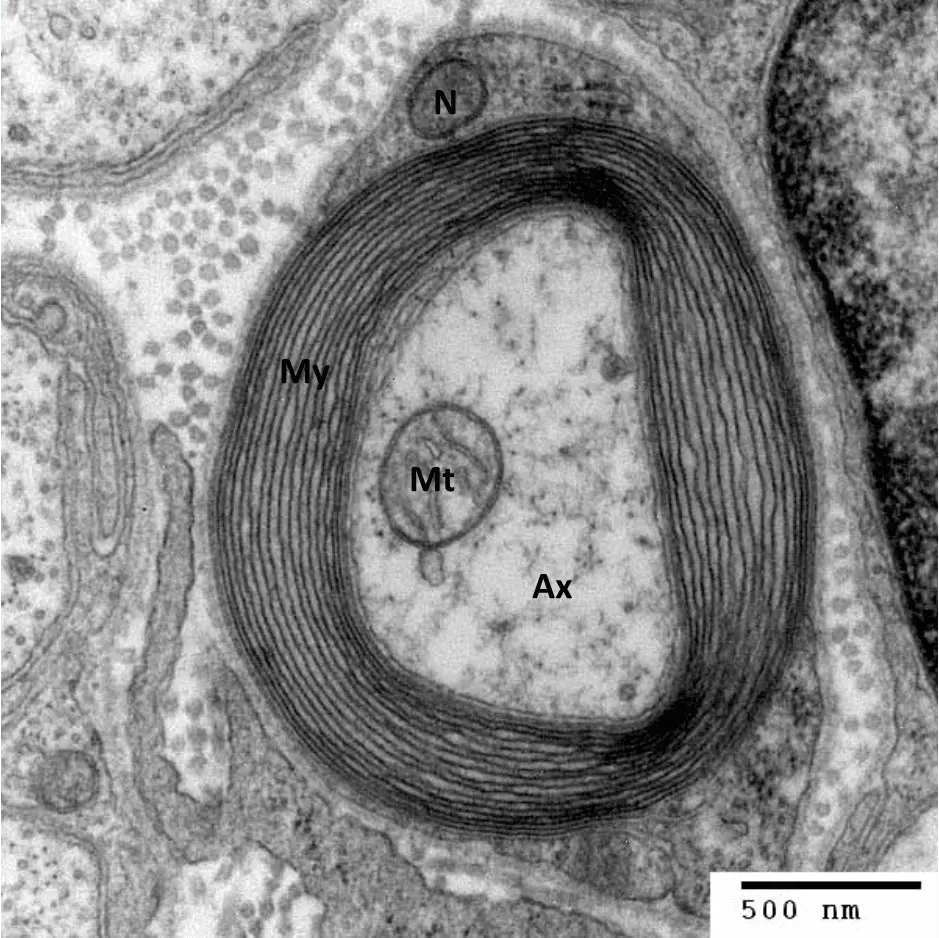
Image - An electron micrograph image showing a transverse section of the myelin sheath
My = Myelin sheath, Mt = Mitochondrion, Ax = Axonal cytoplasm, N = Schwann cell nucleus
Creative commons source by Roadnottaken [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
The myelin sheath acts as an electrical insulator, causing the local current to spread to the next node before the next action potential is generated. This is described as saltatory conduction, because the action potential appears to ‘jump’ from node to node. This is much faster than if action potentials had to be continuously generated along the entire length of axon.
Myelination improves conduction by increasing the length constant. This is achieved by:
- Decreased membrane capacitance (Cm)
- Increased membrane resistance (Rm)
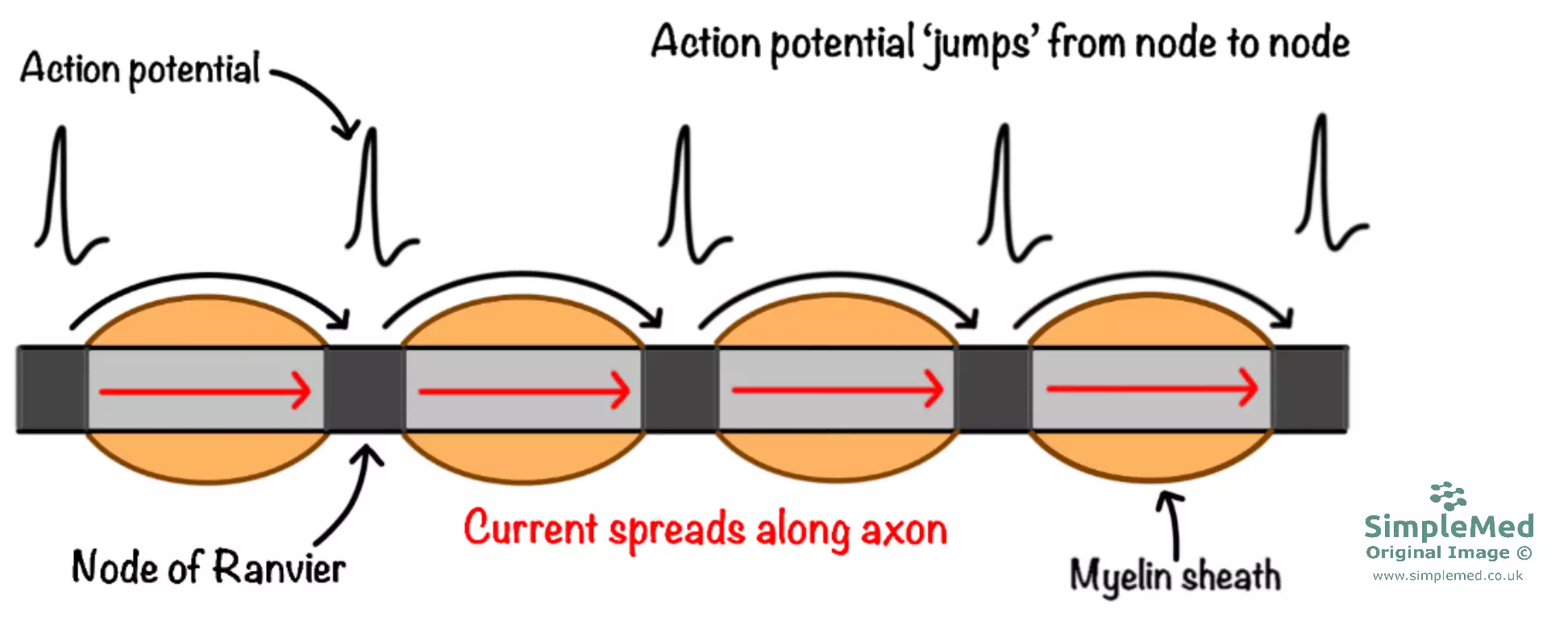
Diagram - Model of a myelinated nerve axon demonstrating saltatory conduction
SimpleMed original by Dr. Joshua Bray
Demyelination diseases, such as multiple sclerosis (MS) and Charcot-Marie-Tooth disease, result from breakdown of the myelin sheath. They are characterised by impaired nervous transmission. Areas of damaged myelin sheath (plaques) have low resistance and high capacitance, so the local current fails to spread to the next node so the next action potential cannot be generated.
The neuromuscular junction is the synapse between a neurone and a skeletal muscle cell. As with all synapses, it involves the release of a neurotransmitter which diffuses across the synaptic cleft and activates receptors on the post-synaptic membrane - at the neuromuscular junction, this neurotransmitter is acetylcholine (ACh).
The process of synaptic transmission at the neuromuscular junction is as follows:
- The action potential arrives at the synaptic terminal and the local depolarisation causes voltage-gated Ca2+ channels to open, resulting in Ca2+ influx.
- Ca2+ binds to synaptotagmin on the pre-synaptic membrane, which brings vesicles containing ACh to the membrane and forms a SNARE complex. The ACh is consequently released via exocytosis.
- ACh diffuses across the synaptic cleft and binds to nicotinic ACh receptors (nAChRs) on the sarcolemma (this area is termed the ‘motor end plate’). Activation of these receptors results in Na+ influx and consequent depolarisation of the end plate. ACh is degraded by an enzyme called acetylcholinesterase (AChE) and its constituents are taken up by the neurone for recycling.
- Local spread of depolarisation across the sarcolemma causes VGSCs to open resulting in action potential generation. The action potential initiates contraction of the skeletal muscle fibre, a process which is mediated by a rise in intracellular Ca2+ (see article on Membrane Transport).
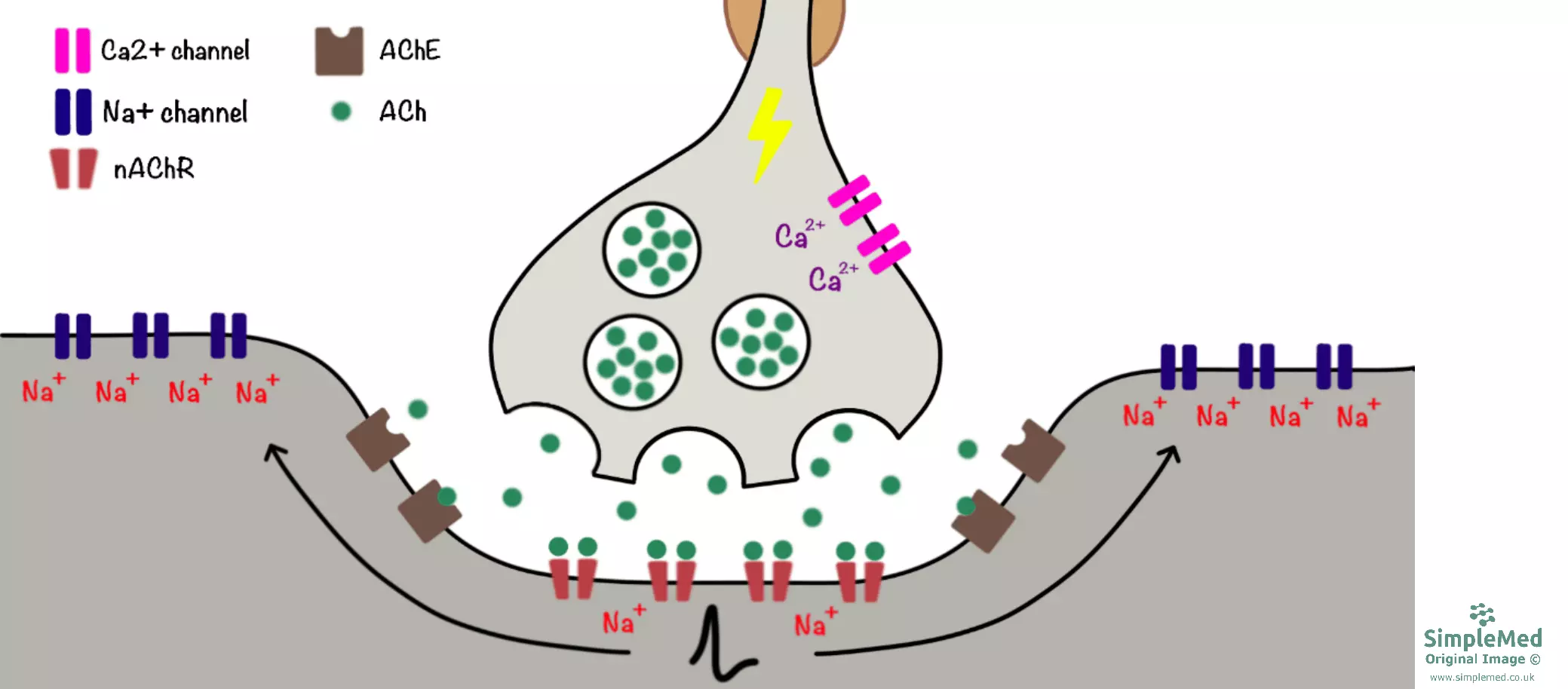
Diagram - Synaptic transmission at the neuromuscular junction
SimpleMed original by Dr. Joshua Bray
Drugs which block action at the neuromuscular junction can be used as muscle relaxants in conjunction with general anaesthesia. They are particularly useful for relaxing the vocal cords to facilitate endotracheal intubation.
Neuromuscular blockers can work via 2 different mechanisms:
- Non-depolarising blockers (e.g. rocuronium bromide) - directly block nAChRs at the motor end plate, thereby preventing depolarisation.
- Depolarising blockers (e.g. succinylcholine) - These drugs activate nAChRs, however the sustained depolarisation causes the surrounding Na+ channels to become inactivated so an action potential cannot be generated.
Myasthenia gravis is an autoimmune disease where autoantibodies target and destroy nAChRs at the neuromuscular junction, so it is harder for the end plate potential to reach the threshold for action potential generation. This disease is characterised by fatigable muscle weakness and is treated using acetylcholinesterase inhibitors such as pyridostigmine.
Reviewed by: Dr. Thomas Burnell
- 18099

