By Dr. Marcus Judge & Dr. Ethan Kavanagh
Next Lesson - Streptococci
Contents
- Introduction
- Classification of Antibiotics
- Mechanism of Action
- Summary List
- Beta-lactams
- Glycopeptides
- Tetracyclines
- Aminoglycosides
- Macrolides
- Quinolones
- Trimethoprim and Sulphonamides
- Antifungals
- Antivirals
- One Last Drug
- Antibiotic Resistance
- Development of Resistance by Mutation
- Horizontal Gene Transfer
- Quiz
Abstract
- Antibiotics broadly work by one of five ways: inhibiting protein synthesis, inhibiting cell wall synthesis, inhibiting cell membrane function, inhibiting nucleic acid synthesis or inhibiting metabolic pathways.
- The main antibiotic families are: beta-lactams (penicillins, cephalosporins, carbapenems, monobactams), tetracyclines, glycopeptides, aminoglycosides, macrolides, polymixins and quinolones.
- There are three main ways that bacteria develop resistance: intrinsic, acquired and adaptive resistance.
Core
Antibiotics, also known as antibacterials or antimicrobials, are a collective group of drugs which are used to treat conditions caused by a variety of bacteria and other organisms. It is important to note that they do not treat viruses (or at least not primary viral infections). Antibiotics come in a range of formulations, from topical creams to intravenous (IV) infusions used for more serious bacterial infections.
Since the discovery of penicillin by Fleming, there are now a wide range of antibiotics available for a doctor to prescribe. When designing an ideal antibiotic or in fact any drug there are a few desirable goals
- A drug with few adverse side effects and few drug to drug interactions - improves patient comfort, compliance and minimises further harm to the patient.
- Specifically toxic - i.e. damages a specific organism and not the patient’s cells and tissues.
- Able to achieve high concentrations at the site of infection.
- A long half-life reduces the need to frequently repeat doses.
- A wide range of formulations - allows for multiple routes of administration and application e.g. oral tablets for home use, IV formula to deliver directly into the bloodstream in hospital.
Half-life is the time taken for the concentration of a drug within plasma to fall to a half of what its initial starting concentration was. This is determined by how quickly the drug is eliminated (clearance) from the plasma and how widely it spreads within the tissues (volume of distribution). The desired length of half life depends on what the drug is being used for. With antibiotics, the duration of activity needs to be appropriate for the infection and the drug’s pharmacokinetic and pharmacodynamic properties, so that effective concentrations are achieved without unnecessary toxicity or dosing. You can imagine this would be good for improving compliance in a patient’s treatment regimen (e.g. only taking 3 tablets instead of 7) and would help in a hospital to reduce the workload of the staff having to re-administer or re-hang IV bags.
We can classify antibiotics based on a number of different criteria, such as:
- Lethal to bacteria (bactericidal) or slows/ stops growth and reproduction (bacteriostatic).
- Attack a wide variety of bacteria (broad spectrum) or specific to only a few types (narrow spectrum).
- The site of action on bacteria - where is the target site and what is the mechanism of action.
- Chemical structure of the antibiotic
These are the four main mechanisms of action for antibiotics. Each family of antibiotics has a common mechanism:
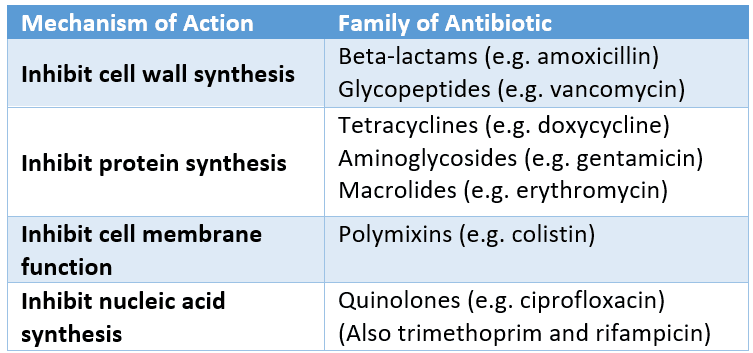
Figure: The mechanisms of antibiotics and the classes of antibiotics that use each mechanism
SimpleMed original by Dr. Ethan Kavanagh
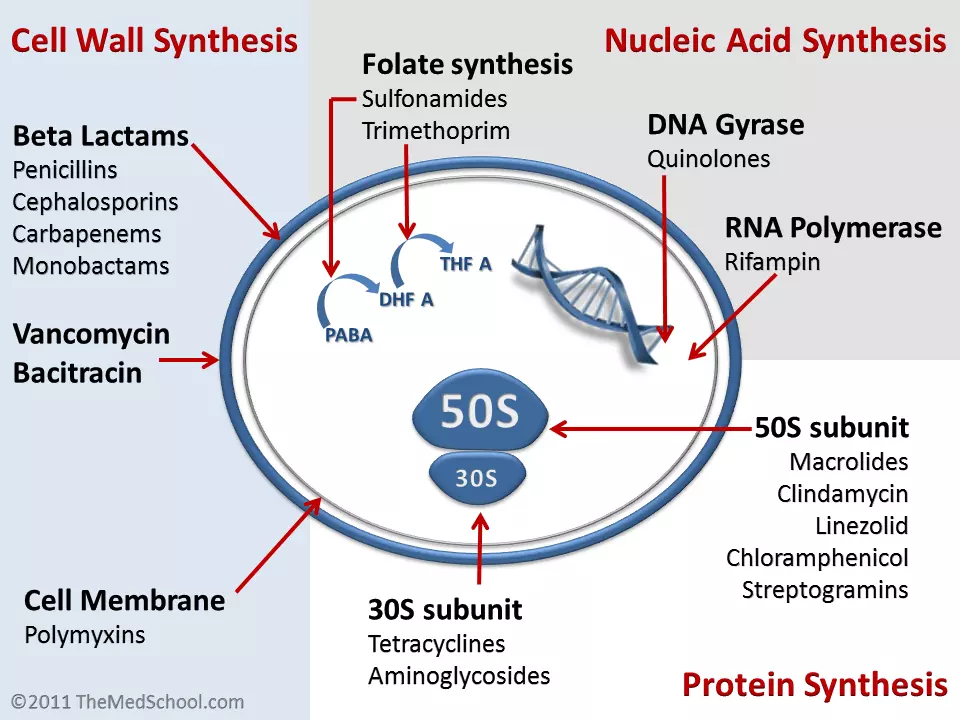
Figure: Shows the mechanism of action of antibiotics and where they act within bacteria
Creative commons source by Kendrick Johnson [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
- Class of antibiotic
- Example within the class
- Example within the class
Please see below the list for further information on specific antibiotics of note
Beta lactams
- Penicillins
- Penicillin/ Benzylpenicillin
- Amoxicillin
- Flucloxacillin
- Co-amoxiclav
- Cephalosporins
- Ceftriaxone
- Carbapenems
- Meropenem
- Imipenem
The rest
- Glycopeptides
- Vancomycin
- Teicoplanin
- Tetracycline
- Tetracycline
- Doxycycline
- Macrolides
- Erythromycin
- Aminoglycosides
- Gentamicin
- Quinolones
- Ciprofloxacin
- Trimethoprim
- Trimethoprim
- Co-trimoxazole
Antifungals
- Polyenes
- Nystatin
- Amphotericin
- Azoles
- Fluconazole
Broad-spectrum antibiotics, particularly certain classes, are associated with a higher risk of C. difficile infection. Risk relates to the antibiotic class and spectrum rather than the first letter of the drug name.
This is an umbrella group that encompasses four families of antibiotics: penicillins, cephalosporins, carbapenems and monobactams.
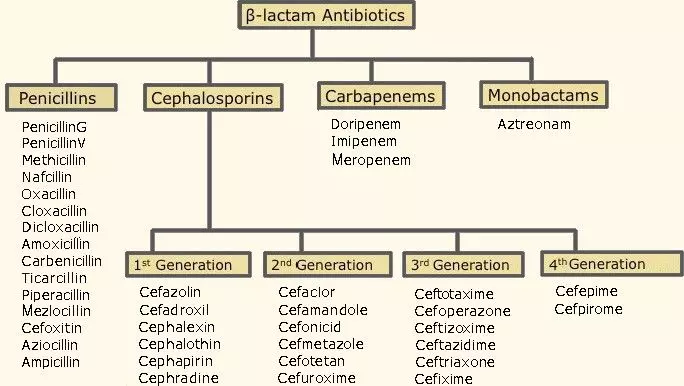
Figure: Shows the Beta-lactam family with examples of antibiotics within each family
Creative commons source by Helito, edited by Dr. Tom Burnell [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
Penicillins
Most penicillins have the suffix “-cillin” so you can mostly tell immediately if an antibiotic is a penicillin. In a variety of different ways, they all interfere with the third and final stage of bacterial cell wall synthesis.
Important penicillins to remember are benzylpenicillin (what is usually referred to as just ‘penicillin’ or Ben Pen), amoxicillin, flucloxacillin and co-amoxiclav.
Penicillins are particularly effective against Gram-positive bacteria (mainly Streptococci) which have a thicker and more exposed cell wall. Activity against Gram-negative bacteria varies by penicillin subclass, with some agents such as amoxicillin having useful Gram-negative coverage. Flucloxacillin is effective against both Staphylococci and Streptococci.
Co-amoxiclav which is a combination of amoxicillin and clavulanic acid (a beta lactamase inhibitor). Amoxicillin’s efficacy versus some bacteria such as Staphylococcus aureus can be reduced (and is a mechanism of resistance) by an enzyme which these bacteria excrete called beta-lactamase. This beta lactamase reduces antibacterial efficacy as it breaks down the antibiotic preventing it from working against the bacteria. Clavulanic acid inhibits beta-lactamase allowing amoxicillin to function for longer before it is broken down and thus making it more effective. This makes co-amoxiclav effective against Staphylococci, Streptococci, Gram-negative bacteria and gives it an effect on anaerobic bacteria as well.
Cephalosporins
There are considered to be five generations of cephalosporins but each of them works by inhibiting cell wall synthesis.
Unlike some penicillins, most cephalosporins have limited activity against anaerobic bacteria, but they are otherwise relatively broad spectrum. Ceftriaxone is useful in treating meningitis because of its good level of activity in the cerebrospinal fluid due to its ability to easily cross the blood-brain barrier.
There is some concern over cephalosporins causing Clostridium difficile infection. C. diff is a commensal bacterium found in your normal gut microbiome that competes for nutrients with other flora. This constant competition prevents C. diff overgrowth. Cephalosporins can also eliminate some of the natural gut flora as well as the pathogenic organism in an infection. This reduces competition allowing for C.diff to proliferate and over grow causing disease.
A commonly taught rule of thumb is that several antibiotics beginning with the letter ‘C’ are associated with an increased risk of C. diff infection. However, this is an oversimplification. The risk relates more to the antibiotic spectrum and impact on gut flora rather than the name itself.
Carbapenems
Meropenem is the main carbapenem that works by inhibiting cell wall synthesis. Alongside Gram-positive and Gram-negative bacteria, it is active against anaerobic bacteria. Meropenem is strongest against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus, though it is not effective against MRSA.
Meropenem has a similar chemical structure to penicillins, but true cross-reactivity is uncommon. It may still be used with caution depending on the severity and type of penicillin allergy.
Vancomycin is the main glycopeptide. It inhibits cell wall synthesis by preventing two subunits from being incorporated into the peptidoglycan matrix that is the core structural component of the cell walls of Gram-positive bacteria. It is active against both aerobic and anaerobic bacteria. It can also affect membrane permeability and RNA synthesis of the bacteria, but the key mechanism is inhibition of cell wall synthesis. Vancomycin does have a narrow therapeutic window, so the patient needs to have therapeutic drug monitoring to ensure that the dose does not become toxic.
Something to remember about vancomycin is that it is a reserved antibiotic for serious Gram-positive infections. There are still many bacteria that are not resistant to it, including multiresistant staphylococci and Clostridium difficile, so it is only given when absolutely necessary to prevent sudden overuse and resistance among bacteria. There are some reports of reduced susceptibility in Staphylococcus aureus and reports of glycopeptide resistant enterococci.
Vancomycin is normally given IV (especially for systemic infections) because it isn’t absorbed via the gut. The exception to this is in the case of a C. diff infection whereby it is given orally because the target is the GI tract.
The other glycopeptide of note is Teicoplanin. This has a larger therapeutic index and is therefore much easier to monitor with the risk of a toxic dose less likely.
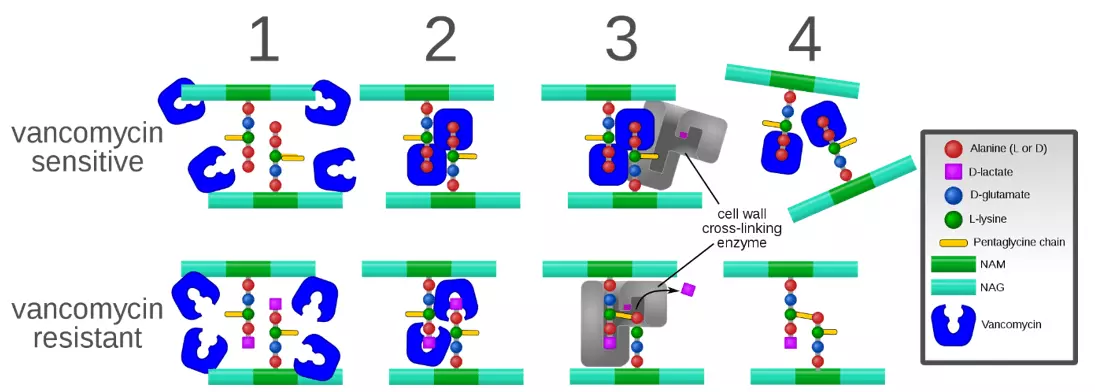
Figure: Shows the process of vancomycin resistance
Creative commons source by Mcstrother [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
The two main tetracyclines are doxycycline and tetracycline. Both are bacteriostatic antibiotics through their action of inhibiting protein synthesis of the bacteria (this holds them in the stationary phase of their growth cycle instead of killing them outright (bactericidal)). Both doxycycline and tetracycline are broad spectrum antibiotics and are a good choice for patients with a penicillin allergy that you are treating for a Gram-positive bacterium.
These antibiotics are effective against illness such as Chlamydia, rocky mountain spotted fever and typhoid fever. They should not be used for Streptococcal infections as up to 44% of S. pyogenes and 74% of S. faecalis have been found to be resistant to tetracyclines. This is why they are decreasing in value except in a number of different illnesses or patients with penicillin allergies (e.g. in leptospirosis as an alternative to erythromycin).
Tetracycline cannot be given to children younger than 8 years old as it can cause permanent discolouration of the teeth among other adverse effects. Bioavailability of tetracycline is 100% via IV, but only 40% via intramuscular (IM).
Aminoglycosides include gentamicin (the main aminoglycoside), amikacin and streptomycin. They work by inhibiting protein synthesis. They have a bactericidal action which is particularly strong against Gram-negative bacteria.
Aminoglycosides are mainly used for Pseudomonas and Enterobacter, while also being effective against some strains of Mycobacteria. They can be used for some Gram-positive bacterial infections but this is uncommon.
Gentamicin has good activity in the blood and urine which is why it is used for an undiagnosed serious systemic infection (when combined with a penicillin or metronidazole). It is normally reserved for Gram-negative sepsis because of its strength and lack of resistance.
Gentamicin does have a couple of issues though. It is nephrotoxic as it attacks the cells of the proximal convoluted tubule in the kidney, but the cells do regenerate - even if the drug is continued to be taken. It also appears to cause non-reversible ototoxicity (damage to the ear). It causes irreversible damage to the hair cells of the cochlea, leading to a loss of high frequency hearing followed by low frequency hearing.
Due to the nephrotoxicity and ototoxicity, patients on gentamicin will need to be monitored (both for symptoms and to ensure the levels of gentamicin within the patient are with the therapeutic window). As such, courses of gentamicin are usually kept as short as possible, often around 7 days, with duration guided by indication and careful monitoring.
Macrolides work by inhibiting protein synthesis. They include erythromycin, clarithromycin and azithromycin. They have a similar spectrum of action to that of penicillins, which makes them a good alternative in patients with a penicillin allergy (for mild Gram-positive infections). This property also makes them useful for penicillin resistant infections, but unfortunately some of those bacteria are becoming resistant to macrolides.
Erythromycin is good for atypical pathogens in pneumonia, Legionnaires’ disease, early syphilis and Chlamydia. What it does not have great activity against is Haemophilus influenzae. A way to work around this is the combination of erythromycin with a sulphonamide which will result in activity against a number of strains of H. influenzae.
Another issue with erythromycin specifically, is that it tends to make patients nauseous, vomit or give them diarrhoea. If a patient has a mild infection then a lower dose can be prescribed, but when the infection is more serious (such as with a Legionella infection/pneumonia) then a higher dose needs to be prescribed. This is where the good and bad need to be compared to see which possible treatment plan has the best effect with the least repercussions for the patient.
Both azithromycin and erythromycin have activity against many Gram-positive bacteria, particularly Streptococcus species, but azithromycin makes up for erythromycin’s weaker activity against H. influenzae with better Gram-negative cover as a stand-alone treatment. Clarithromycin was derived from erythromycin, with the former being a little more effective than the latter. Clarithromycin is used to treat Helicobacter pylori infections (along with metronidazole and proton pump inhibitors).
Quinolones are a collection of antibiotics that work by inhibiting bacterial replication through inhibiting DNA gyrase. Within the UK, the only quinolones that are available to prescribe are fluoroquinolones so they are the only ones that will be touched on in this article.
Ciprofloxacin is best against Gram negative bacteria such as Escherichia coli, Salmonella and Shigella. It is only moderately active against Gram-positive bacteria such as Streptococcus, and a lot of Staphylococci are resistant to quinolones so make sure not to use them in MRSA infections. Resistance amongst other bacteria to quinolones is also increasing, and there is a risk of a C. diff infection for those taking quinolones.
Ciprofloxacin is given as an eye drop, ointment, orally and IV. It is used for UTIs, some GI infections and septicaemia due to susceptible organisms. It is not routinely used for gonorrhoea in the UK because of resistance, unless susceptibility is confirmed. Currently, it is being investigated for its use against malaria, cancers and AIDS.
Trimethoprim and Sulphonamides
Trimethoprim works to inhibit dihydrofolate reductase, an enzyme that is important in the folic acid cycle. Dihydrofolate reductase’s normal role is the conversion of dihydrofolic acid to tetrahydrofolic acid in the thymidine synthesis pathway. It is often combined with a sulphonamide, sulfamethoxazole, which are bacteriostatic antibiotics. As a side note, sulfamethoxazole acts on dihydrofolate synthetase, which is involved further upstream in the folic acid pathway. Combined together, you get co-trimoxazole which is more effective than either of its standalone components. Co-trimoxazole is used more for uncomplicated pyelonephritis and can be used in the case of COPD exacerbation.
Trimethoprim alone is commonly used to treat UTIs, acne, prostatitis and shigellosis. One other condition that co-trimoxazole is used to treat is pneumocystis pneumonia. As it interferes with folic acid synthesis, trimethoprim should not be prescribed to a pregnant patient, particularly during the first trimester when folic acid supplements are often given to help with fetal development.
There are two main families of anti-fungals; azoles and polyenes.
Fluconazole is an example of an azole. It can be used for both systemic and superficial fungal infections but, although there are a number of other azole drugs, fluconazole has relatively smaller chance of side effects. Some resistant strains of Candida have been detected, so a susceptibility test will need to be done first before prescribing. If a patient has an Aspergillus infection, then posaconazole is a good alternative.
Fluconazole works by inhibiting ergosterol synthesis in the fungal cell membrane, impairing membrane function and growth. Previously, there was some concern that human cytochrome P450 enzymes would be targeted, however, fluconazole has been shown to be selective for fungal cytochrome P450 enzymes rather than human enzymes.
Polyenes do not have a nice nomenclature system like the azole antifungals. The mechanism of action of polyenes is to inhibit cell membrane function. Nystatin is prescribed as a topical treatment for Candida infections, whereas amphotericin is prescribed intravenously for systemic fungal infections. Nystatin can be toxic when given via IV.
Aciclovir works by inhibiting viral DNA polymerase. The viruses aciclovir is brought in to fight are Herpes simplex and Varicella zoster virus. The former is responsible for genital herpes, with the latter causing chickenpox and shingles. A particular enzyme that Herpes simplex and Varicella zoster encode phosphorylates aciclovir into its active form and it can then begin to work.
A big debate over the use of aciclovir is in Bell’s palsy. Essentially, some clinicians will prescribe aciclovir while others will combine it with steroids and other clinicians prescribe nothing at all. This is a great example where you can turn to the evidence behind the use of aciclovir (and steroids for that matter) to treat Bell’s palsy. If you check out the facts, you’ll see that:
- A large proportion of cases of Bell’s palsy will resolve on their own over time.
- Prescribing aciclovir on its own has not been shown to provide significant benefit in most cases.
- There is only a small difference made when co-prescribing steroids and aciclovir.
So, for a small difference which may or may not help the patient, is prescribing the combination therapy a good idea? Not only is the patient being on medication one factor, but the cost of the drugs on the NHS is another factor. This debate is beyond the aims of this article, but still an interesting point.
Oseltamivir, sold under the brand name Tamiflu®, is another antiviral but it works by inhibiting neuraminidase. It is used specifically as prophylaxis and treatment of patients that are of at least two weeks of age and have had symptoms of Influenza A and/or Influenza B for no more than forty-eight hours. It is almost like the reverse of aciclovir as oseltamivir starts off in its pro-drug form as a phosphate rather than being phosphorylated so that it can be activated.
One final drug that requires mentioning is metronidazole. Its main targets are anaerobic bacteria and protozoa.
It is used for a whole host of infections from H. pylori, fistulae in Crohn’s disease, bacterial vaginosis, pelvic inflammatory disease, ulcerative gingivitis, amoebiasis, trichomoniasis, giardiasis and Clostridium difficile.
Normally, for an anaerobic infection, metronidazole is prescribed for 7 days. When a patient has C. diff, treatment is usually given for around 10 days. Current guidelines recommend oral vancomycin or fidaxomicin rather than metronidazole, and treatment is not combined with proton pump inhibitors or macrolides for this indication.
Unfortunately as a consequence of natural selection and random mutation bacteria can easily develop resistance to antibiotics. There are three main types of antibiotic resistance:
- Intrinsic - the bacteria have no target for the drug to work on e.g. a penicillin can’t act on a microbe without a cell wall as this is its site of action.
- Acquired - this is done either through mutation or horizontal gene transfer.
- Adaptive - The bacteria are only resistant when exposed to an antibiotic as they adapt to the environment. This form of resistance is unstable, therefore when the antibiotic is removed, the bacteria become susceptible once again. (This is a reason for why efficacy of an antibiotic regimen may fall with treatment.)
Acquired resistance is the most commonly referred to mechanism of antibiotic resistance and is linked with antibiotic overuse.
Development of Resistance by Mutation
Resistance by mutation develops in several simple steps.
Within a population of bacteria, through random mutation, some bacteria will develop antibiotic resistant traits. This is of no real concern as these bacteria are few and far between within the larger population. On the introduction of a selection pressure i.e. the introduction of an antibiotic, only these resistant cells will survive and are thus selected for. This causes issues now as because now only the resistant bacteria are alive to reproduce. This leads to a population which is entirely resistant to the antibiotic, compared to one with only a few resistant individuals.
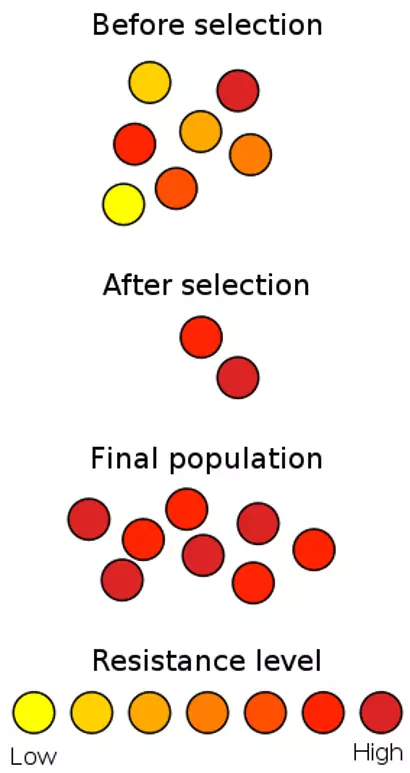
Figure: Shows how antibiotic resistance develops through natural selection as only the high resistance bacteria are selected for
Creative commons source by Wykis [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
As you may recall, horizontal transmission is from one sibling to another, this same terminology when used in the sense of horizontal gene transfer refers to the sharing of genetic information between individuals in the same generation. Horizontal gene transfer allows for luxury genes (in the form of plasmids) to be transferred between individuals. Some luxury genes confer antibiotic resistance attributes and as a result can lead to the spread of antibiotic resistance, even between different species.
Editors note: It is unrealistic to be able to remember every single antibiotic and its uses but it is helpful to be familiar with some examples, mechanisms and principles of antibiotics which this article hopes to achieve. For practitioners in the UK the BNF (link) is the definitive guide for all drugs (please see our disclaimer at the foot of the page) and should be considered along with your local antibiotics guidelines.
Edited by: Dr. Ben Appleby and Dr. Thomas Burnell
- 34803

