Next Lesson - Protein Function in Oxygen Transport
Contents
- Role of proteins
- Amino acids
- Classification of amino acids
- pKa and R groups of amino acids
- Structure of proteins
- Isoelectric point of proteins
- Conjugated proteins
- Secondary structure of a protein
- Tertiary structure of a protein
- Water soluble proteins
- Membrane proteins
- Quaternary structure of a protein
- Protein denaturation
- Misfolding of proteins
- Quiz
Abstract
- Proteins are formed of monomers called amino acids. There are 20 different amino acids that form polymers in different combinations to form different proteins.
- An amino acid is classified based on the R group present in its structure. This R group can affect the overall chemical properties of the amino acid.
- Amino acids join together by forming a peptide bond and by binding together.
- Proteins can have primary, secondary, tertiary and even a quaternary structure in some cases.
Core
Proteins are found throughout the human body are play an important role in all biochemical processes:
- Catalysts - enzymes
- Transporters - haemoglobin for transport of oxygen
- Structural support - collagen
- Immune system - immunoglobins
- Ligands in cell signalling - hormones and neurotransmitters
- Ion channels and receptors - for hormones and neurotransmitters
Proteins are polypeptides, made up of covalently linked amino acids (monomers). The amino acid sequence of a protein is encoded by a gene. Therefore, the nucleotide sequence of a gene determines the sequence of amino acids in the protein. The final polypeptide chain folds into a highly-specific and intricate three-dimensional structure, which is determined by the sequence of amino acids.
There are different names to describe the sizes of peptides & proteins:
- Peptides or oligopeptides = relatively small polymers with a low number of amino acid residues.
- Polypeptides or proteins = large polymers with large numbers of amino acid residues.
Amino Acids Chemical Structure
Amino acids are the building blocks of proteins as the monomers that the final protein structure is made out of. They all have a general structure of central carbon atom covalently bonded to an amino group (NH2), carboxyl group (COOH), a hydrogen atom and distinctive R group (the side chain).
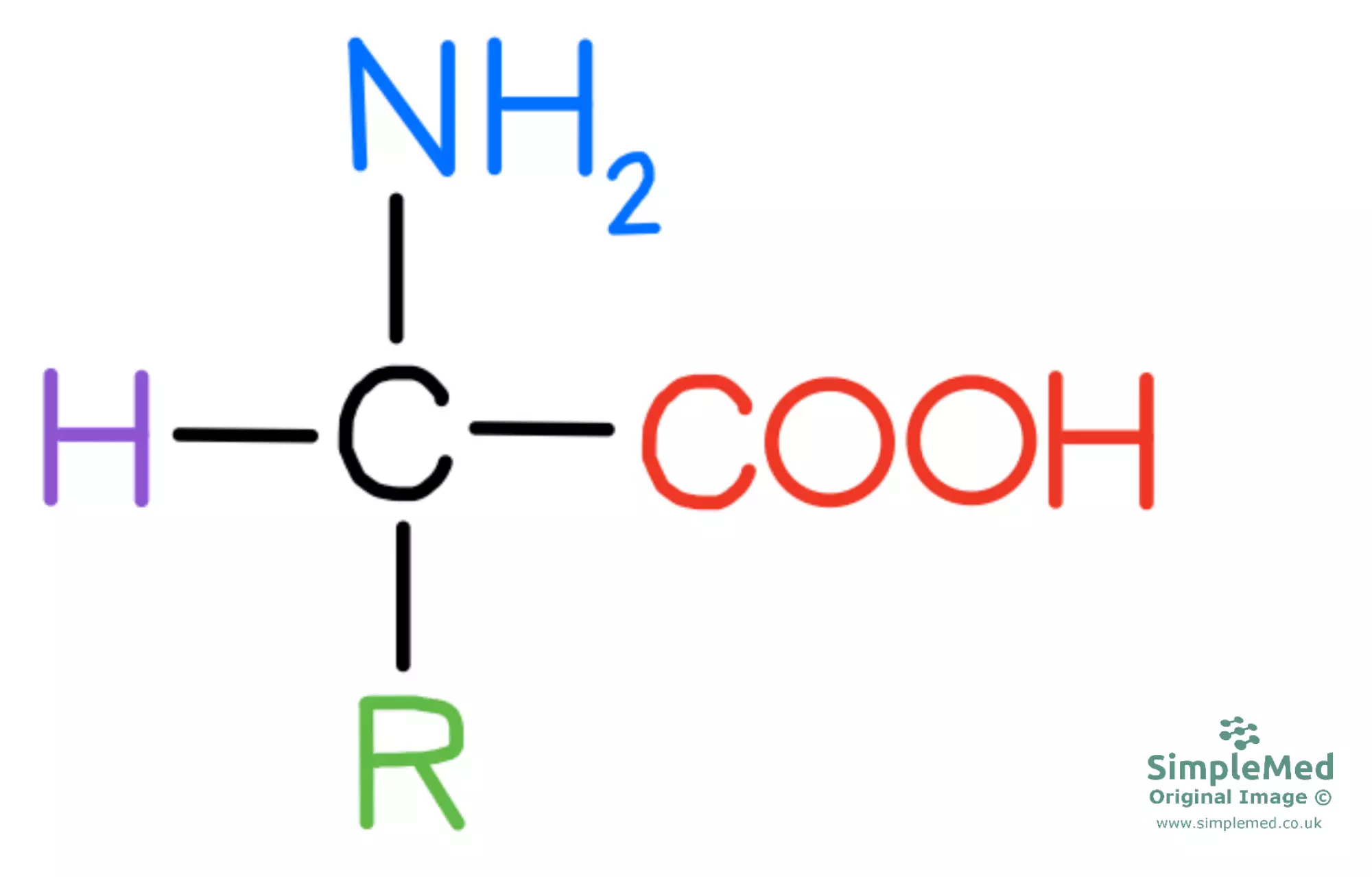
Diagram - The general structure of an amino acid
SimpleMed original by Dr. Peter Parkinson
Amino acids become ionised because the amino group can act as a base (proton acceptor) and the carboxyl group can act as an acid (proton donor). In aqueous solution, their protonation states are determined by acid-base equilibria with the surrounding environment. The amino group can act as a proton acceptor because the nitrogen ion has a pair of electrons in its outer shell that are not involved in bonding, which can bond to the proton. This means that the amino group goes from NH2 to NH3+, and the carboxyl group transitions from COOH to COO-.

Diagram - How the amino and carboxyl groups exchange a H+ ion to become charged
SimpleMed original by Dr. Peter Parkinson
When the amino acid has one negative charge and one positive charge, the net charge of the amino is zero and this is known as a zwitterion. Depending on the pH of the solution, the amino acid can exist as a cation (overall positive charge) or as an anion (overall negative charge).

Diagram - The structure of a zwitterion
SimpleMed original by Dr. Peter Parkinson
A peptide bond forms between individual amino acids to connect them. The remaining part of each amino acid now left in the amino acid chain is called the amino acid residue. The peptide bond is formed in a condensation reaction as a single molecule of H2O is produced with each peptide bond formed.

Diagram - The general appearance of an amino acid residue
SimpleMed original by Dr. Peter Parkinson
Amino acids are classified based on their R group which contributes to the overall function of the protein. The acid-base behaviour of the protein is determined by the R groups of the amino acids.
Chemical properties of amino acid:
- Polar (hydrophilic) - able to form hydrogen bonds and can be further classified into positive, negative or neutral charge
- Non-polar (hydrophobic)
- Acidic
- Basic
- Neutral
Physical properties of amino acid:
- Aliphatic - R group just contains carbon and hydrogen atoms
- Aromatic - R group with a cyclic, planar molecule which has great stability e.g. R group on phenylalanine

Diagram - The chemical structure of phenylalanine with its R group in red
SimpleMed original by Dr. Peter Parkinson
pKa and R Groups of Amino Acids
pKa is the pH at which an ionisable group is 50% protonated and 50% deprotonated, and is a measure used to indicate the strength of an acid. Whether an amino acid is positively or negatively charged, depends on the pKR (pK of the side group) value of the side group.
Positively charged R groups with pKR values:
- Lysine - 10.5
- Histidine - 6.0
- Arginine - 12.5
Negatively charged R with pKR values:
- Glutamate - 4.3
- Aspartate - 2.8
If the pH value of the solution < pKR value then the side group will be protonated (addition of hydrogen ions).
If the pH value of the solution > pKR value then the side group will be de-protonated (loss of hydrogen ions).
Examples:
- Lysine has a pKR of 10.5, therefore at physiological pH (7.4) the R group of lysine will be protonated.
- Aspartate has a pKR of 2.8, therefore at physiological pH (7.4), the R group of
aspartate will be de-protonated.
In summary: the amino acid wants to get the pH closer to its pKa - if the solution has a lower pH (meaning there are free protons) the amino acid will accept them and become protonated to try to raise the pH closer to its pKa. If the solution has a higher pH (meaning there are less free protons) the amino acid will donate its protons and become de-protonated to try to lower the pH closer to its pKa.
Definitions:
- Primary structure = linear amino acid sequence of polypeptide chain.
- Secondary structure = local folding of polypeptide backbone due to interactions between atoms of the backbone excluding R groups. These structures can either be alpha helix or beta-pleated sheet and are held in shape due to hydrogen bonds formed within the structure.
- Tertiary structure = the overall three-dimensional configuration of a protein. It forms based on the interaction between the R groups of the amino acids within the protein.
- Quaternary structure = the interaction between multiple polypeptides and prosthetic groups to form a multi-subunit protein, e.g. haemoglobin which is made up of multiple polypeptide chains and haem prosthetic groups containing iron atoms.
The bond formed by linking two amino acids together is called a peptide bond and the reaction to form this bond is a condensation reaction - removal of a molecule of water.

Diagram - The formation of a peptide bond by a condensation reaction
SimpleMed original by Dr. Peter Parkinson
Characteristics of the peptide bond include:
- Planar - the carbon, hydrogen, oxygen and nitrogen atoms all lie in the same plane.
- Rigid - the carbon-nitrogen bond in the peptide bond has partial double-bond characteristics causing the bond to be unable to rotate. This helps to contribute to the atoms being in the same plane.
- Exhibits a trans conformation - the carbon - R group bonds, of each amino acid residue are found on opposite sides of the peptide bond. The trans conformation reduces steric hindrance between side chains; flexibility of the polypeptide arises from rotation around bonds adjacent to the peptide bond.
The bonds either side of a peptide bond are able to rotate, allowing flexibility within the 3-D structure of the protein.
The way in which the polypeptide chain folds and the physical characteristic of the protein is determined by the sequence of the amino acids.
The overall structure of a protein determines the overall function of the protein.
Isoelectric point (pI) of a protein is the pH at which there is no overall net charge amongst the structure of the protein.
Basic proteins:
- pI > 7
- The protein contains many positively charged/basic amino acids - based on R groups and they will be protonated.
Acidic proteins:
- pI < 7
- The proteins contains many negatively charged/acidic amino acids.
Examples of proteins and their pI:
- Pepsin (protease found in the stomach) - pI of < 1.
- Haemoglobin (protein found in red blood cells that carries oxygen) - pI of 6.8.
- Cytochrome C (protein that carries electrons in the mitochondria in process of oxidative phosphorylation) - pI of 10.7.
Proteins can be covalently bonded to chemical components as well as amino acids. Examples:
- Lipoproteins - lipid non-covalently associated with protein. E.g. LDL and HDL.
- Glycoproteins - carbohydrate covalently linked to protein E.g. Immunoglobulin.
- Phosphoproteins - phosphate group covalently linked to protein. E.g. casein in milk.
Secondary Structure of a Protein
The bonds involved in the secondary structure of a protein are hydrogen bonds.
- Polypeptide chain folded in a right-handed spiral conformation.
- The structure is stabilised by hydrogen bonds between N-H of one amino acid residue and C=O group of an amino acid residue four residues away.
- Not every polypeptide chain forms an alpha helix structure.
- Small, hydrophobic residues (alanine & leucine) are strong helix formers.
- Example: Ferritin (iron storage protein) - majority of the protein is alpha helix structure.

Diagram - The structure of an alpha helix
Creative commons source by Linnikh [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
- In beta-pleated sheets, the polypeptide chains are nearly fully stretched out which allows the R-groups to alternate between opposite sides of the chain.
- When there are two or more of these stretched out chains (beta strands) side by side, hydrogen bonds can form between the strands to give an almost 2-D sheet that is pleated.
- Alternate beta strands can either run in same direction (parallel) or in opposite directions (anti-parallel).
- Example: Receptor on a T cell.

Diagram - An anti-parallel beta-pleated sheet
Creative commons source by Roland.chem [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
Tertiary Structure of a Protein
The tertiary structure of a proteins describes the three-dimensional structure of a polypeptide chain which bonds will form within the molecule to achieve the maximum stability of the structure. There are a number of forces that can hold the polypeptide chain in its tertiary structure and these form from interactions between the R groups of the amino acids in the polypeptide chain.
The forces include:
- Hydrogen bonds.
- Ionic bonds - between R groups of opposite charge.
- Hydrophobic interactions - non-polar, hydrophobic R groups cluster together within the inside of the protein molecule.
- Disulphide bonds - covalent bonds formed between cysteine amino acid residues. These are the strongest bonds that contribute to the tertiary structure of a protein.

Diagram - The bonds formed in the tertiary structure of a protein
Creative commons source by WikiComTD, edited by Dr. Peter Parkinson [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
A protein’s overall tertiary structure indicates its function. There are two broad classifications, namely fibrous or globular proteins.
- Have a role of support, protection and shape.
- Made up of single type of repeating secondary structure & long strands or sheets.
- Example: Collagen.
- Have a role of regulation and catalysis.
- Made up of several types of secondary structure.
- Form compact shapes.
- Example: Enzymes such as alcohol dehydrogenase.
When a polypeptide chain folds, the hydrophobic (non-polar) side chains are buried in the middle of the protein while the hydrophilic (polar) side chains are left on the surface to interact with water molecules when the protein is placed in an aqueous solution.
Quaternary Structure of a Protein
Some proteins are made up of two or more polypeptide chains that associate together; this is the quaternary structure of a protein.
One example of a protein with quaternary structure is haemoglobin. A single molecule of haemoglobin is made up of four subunits - 2 alpha chains and 2 beta chains that interact with each other. As well as the four polypeptide chains, there is an inorganic prosthetic group, haem group, which contains iron. The iron ion is crucial for binding oxygen to be carried around the body.
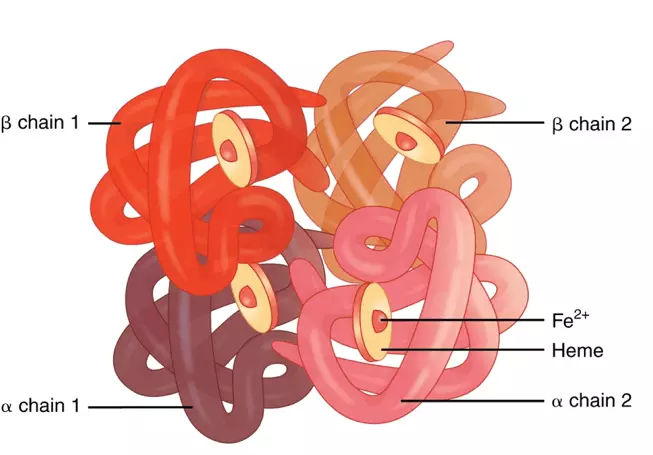
Diagram - The quaternary structure of haemoglobin
Creative commons source by OpenStax College [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
Another example is collagen. Collagen is a fibrous protein formed of three polypeptide chains wound around each other with hydrogen bonds between the chains maintaining the structure. Each chain is relatively long in length therefore there are many hydrogen bonds formed which give the molecule great strength. The collagen molecules form covalent cross links with other collagen molecules to further increase the strength of the protein and the cross links are staggered to increase stability of the molecule.
Collagen is further discussed in our protein targeting & collagen biosynthesis article.

Diagram - The quaternary structure of collagen
SimpleMed original by Dr. Peter Parkinson
Any disruption to the protein’s structure is described as denaturation and involves breaking any of the forces that hold the structure of the protein together.
Heat
- Heat increases the kinetic energy within the molecule and leads to the molecules vibrating rapidly causing disruption to non-covalent interactions such as hydrogen and ionic bonds.
- Extreme heat is used in the process of sterilisation to denature proteins in microorganisms leading to their destruction.
pH
- A change in the pH of the solution the protein is placed in, will affect the ionisation states of the amino acids present within the polypeptide.
- This alteration will change the ionic and hydrogen bonds present within the protein.
Organic Solvents (Detergents)
- Organic solvents have hydrophobic and hydrophilic sides which are attracted to hydrophilic and hydrophobic amino acids present within a polypeptide structure.
- As these amino acids normally help to keep the protein structure together, the detergent acts to pull the protein apart and denature the protein’s shape.
Proteins within the human body can become misfolded for a number of reasons such as genetic defects and environmental factors. These misfolded proteins can then lead to diseased states.
In Alzheimer’s disease, one misfolded form of beta-amyloid protein (breakdown product of amyloid precursor) is thought to be toxic in the brain in abnormal levels as the protein clumps together to form neural plaques. There is also abnormal accumulation of tau proteins, which are normally involved in stabilising the cytoskeleton of the neurone. The accumulation of tau proteins disrupts the normal functioning of neurons, contributing to impaired transport mechanisms and synaptic communication. The neural plaques and tau proteins can affect neural function in the brain and eventually lead to neural cell death.

Image - Beta amyloid proteins (seen in brown) and tau proteins (seen in blue) in Alzheimer's Disease
Creative commons source by National Institute on Aging, NIH [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
Edited by: Dr. Ben Appleby
Reviewed by: Dr. Thomas Burnell
- 13331

