Next Lesson - HPA and Growth Hormone
Abstract
- The adrenal glands are situated above the kidneys, and consist of a capsule, a cortex, and a medulla.
- Aldosterone is the most abundant mineralocorticoid in the body, and has a main role in the control of blood pressure
- Cortisol is the most abundant glucocorticoid in the body, and has a wide range of systemic effects
- The adrenal medulla is a modified sympathetic ganglion of the autonomic nervous system containing chromaffin cells, which functions to synthesise adrenaline and noradrenaline
- Chronic adrenal insufficiency can lead to Addison’s disease
Core
The adrenal glands are situated above the kidneys. They consist of (from peripheral to central) a capsule, a cortex, and a medulla.
The adrenal cortex can be sub-divided into the zona glomerulosa, zona fasiculata, and zona reticularis, which all function to produce different types of corticosteroids, outlined below:
- Zona glomerulosa:produces mineralocorticoids, for example aldosterone.
- Zona fasciculata:produces glucocorticoids, for example cortisol, corticosterone, and cortisone.
- Zona reticularis:produces glucocorticoids and androgens, for example dehydroepiandrosterone, androstenedione, testosterone, and oestrogens.
A useful saying to help you remember the different layers of the adrenal cortex and what they produce is 'salt, sugar, sex; the deeper you go the sweeter it gets'!
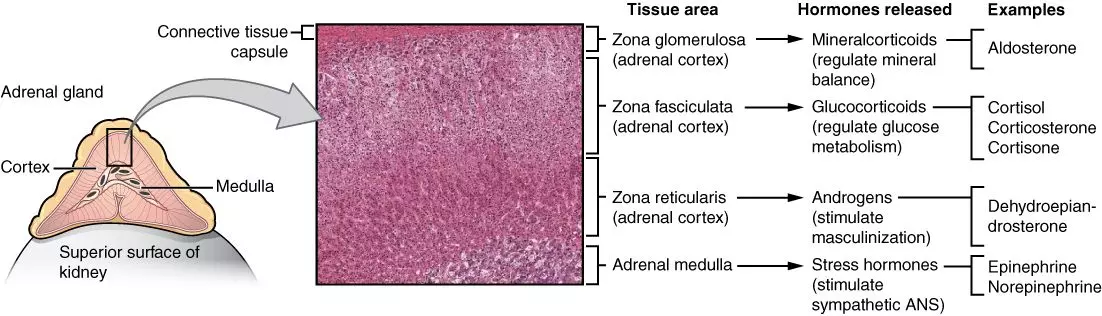
Diagram: Shows the different layers of the adrenal gland histologically and the hormones each layer produces.
Creative commons source by OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)]
Corticosteroids are an example of steroid hormones that are produced in the adrenal cortex (hence the cortico- in the name). Steroid hormones are synthesised from cholesterol and are lipid soluble hormones that bind to nuclear receptors. They mainly act through modulating gene transcription.
Corticosteroids enter cells through simple diffusion across the plasma membrane, where they bind to glucocorticoid receptors. This binding causes the dissociation of chaperone proteins from the receptor (for example heat shock protein 90), allowing the receptor-ligand complex to translocate to the nucleus, where receptor dimerisation occurs and the complex binds to glucocorticoid response elements (GREs) on DNA. In summary, corticosteroids bind to glucocorticoid receptors, which dissociates chaperone proteins, causing translocation to the nucleus and binding to a nuclear receptor.
Aldosterone is the most abundant mineralocorticoid in the body. It is transported in the blood bound to serum albumin and transcortin to a lesser extent.
The main function of aldosterone is the regulation of plasma [Na+] and [K+], in order to control arterial blood pressure. It achieves this through acting on the distal tubules and collecting ducts of the kidney nephrons, where it promotes Na+ reabsorption and K+ excretion through the Na+/K+ ATPase pump, which causes an increase in water reabsorption. This consequently causes an increase in blood volume and therefore increases blood pressure. Aldosterone release is mainly controlled by the Renin-Angiotensin-Aldosterone-System (RAAS), outlined below:
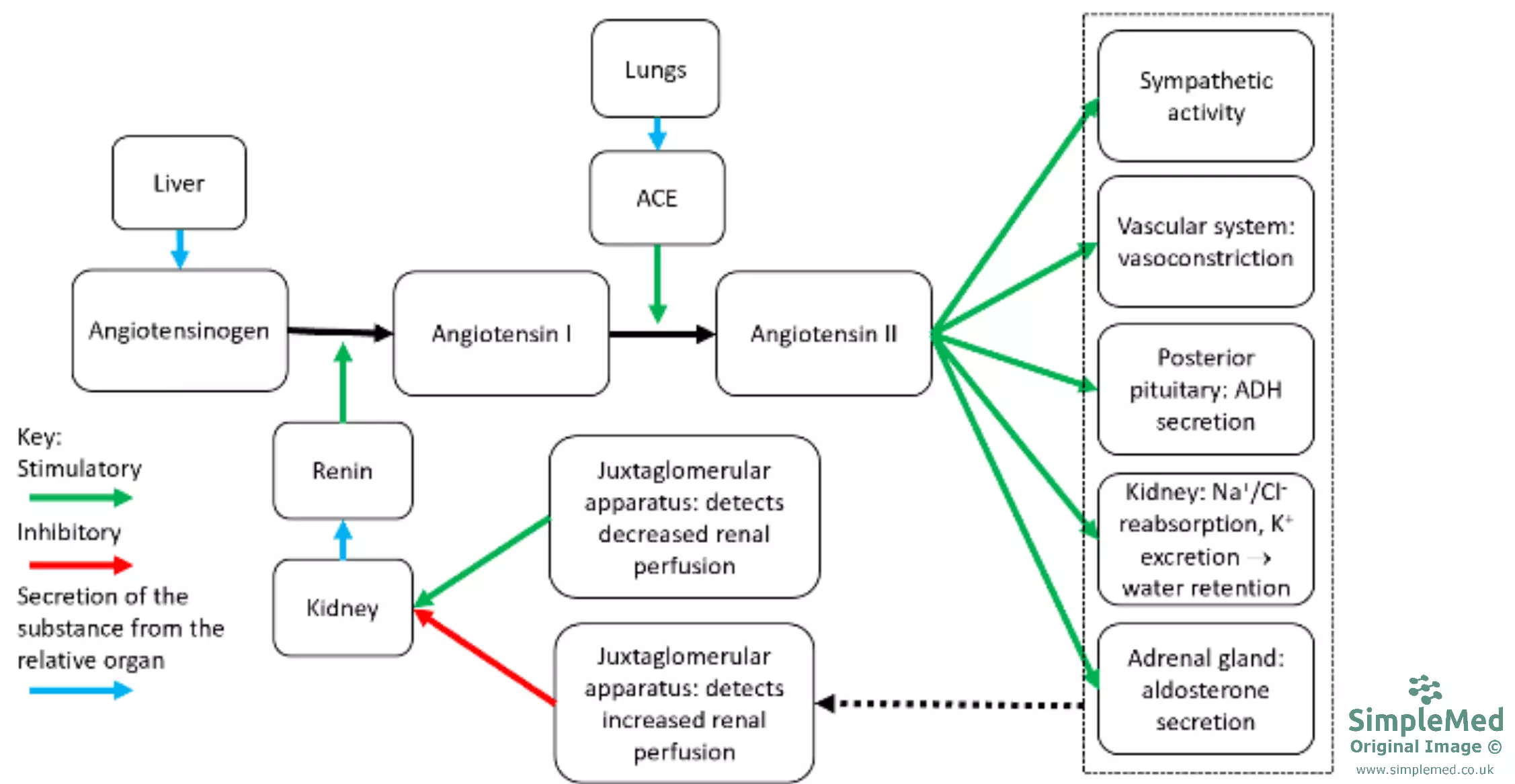
Diagram: Shows an overview of the Renin-Angiotensin-Aldosterone-System. The RAAS is covered in the Cardiovascular Article Hypertension.
SimpleMed Original by Dr. Jenny Hubball
Hyperaldosteronism is defined as a condition in which there is excessive production of aldosterone. This can be further divided into primary and secondary hyperaldosteronism:
- Primary hyperaldosteronism: excess aldosterone production due to a defect in the adrenal cortex.
- The main defect of the adrenal cortex which causes the hyperaldosteronism is bilateral idiopathic adrenal hyperplasia, but an aldosterone-secreting adrenal tumour (Conn’s syndrome) can also be the cause.
- Secondary hyperaldosteronism: excess aldosterone production due to overactivity of the RAAS.
- Overactivity of the RAAS can be caused by a renin-producing tumour, or renal artery stenosis.
The difference between primary hyperaldosteronism and secondary hyperaldosteronism is that the primary condition produces low renin levels (there is a high aldosterone:renin ratio), and the secondary condition produces high renin levels (there is a low aldosterone:renin ratio).
Signs and symptoms of hyperaldosteronism include:
- High blood pressure
- Left ventricular hypertrophy
- Hypernatraemia
- Hypokalaemia
Treatment of hyperaldosteronism depends on the cause. Surgical treatment can be used for aldosterone-secreting adenomas, or spironolactone (a mineralocorticoid receptor antagonist) can be used for other causes.
Cortisol is the most abundant corticosteroid and constitutes approximately 95% of glucocorticoids. It is transported in the blood bound to transcortin. The levels of cortisol are highest in the morning, and cortisol levels fluctuate during the day in a diurnal rhythm.
Cortisol is released from the kidneys in response to adrenocorticotropic hormone (ACTH) released from the anterior pituitary gland. ACTH is released in response to corticotropin-releasing hormone (CRH), released from the hypothalamus. CRH release is controlled through negative feedback to the anterior pituitary and hypothalamus, which inhibits CRH and ACTH release.
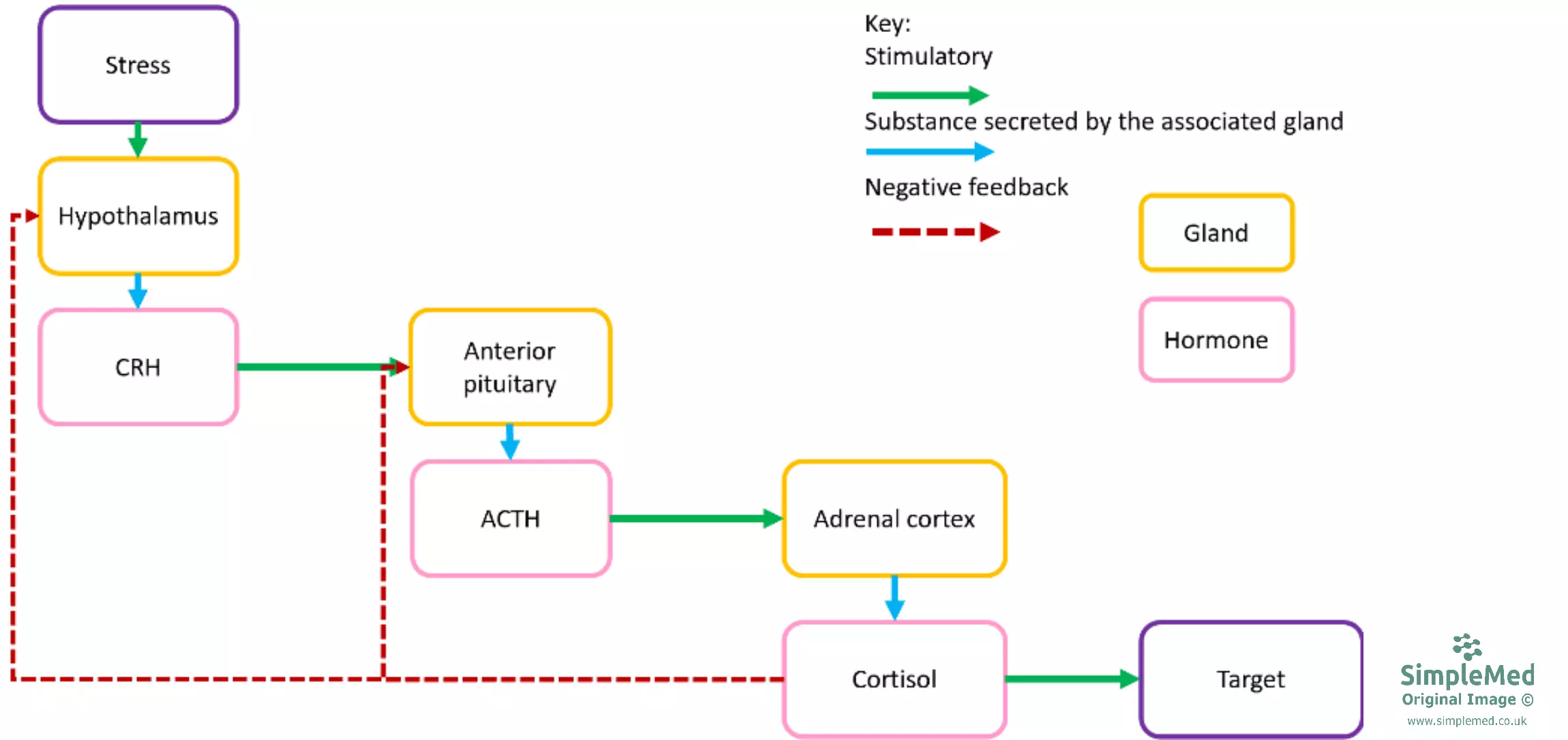
Diagram: Shows the negative feedback mechanism in the control of cortisol production.
SimpleMed Original by Dr. Jenny Hubball
Cortisol has a wide range of systemic effects:
- Catabolic effects:
- Increases protein breakdown in muscle.
- Increases lipolysis in fat.
- Increases gluconeogenesis in the liver.
- In the muscle, cortisol inhibits insulin-induced GLUT4 translocation, preventing glucose uptake by the muscle and having a glucose-sparing effect by increasing blood glucose concentration.
- Resistance to stress:
- Increases supply of glucose in the blood.
- Raises blood pressure through increasing the sensitivity of blood vessels to vasoconstrictors.
- Anti-inflammatory effects:
- Inhibits macrophage activity
- Inhibits mast cell degranulation.
- Depression of the immune response:
- Cortisol is prescribed to organ transplant patients for this reason.
Steroid receptors form part of a family of nuclear DNA binding proteins (including thyroid and vitamin D receptors). They all have three main regions, a hydrophobic hormone-binding region, a DNA-binding region rich in cysteine and basic amino acids, and a variable region. There is sequence homology in the hormone binding regions of the receptors (meaning they are similar), and the percentage homology of the hormone binding region of the glucocorticoid receptor with the mineralocorticoid, androgen, oestrogen and thyroid receptors decreases respectively. This means that cortisol will bind to the mineralocorticoid and androgen receptors with low affinity, which may become significant when high levels of the hormone are present.
Cushing's Syndrome is caused by chronic excessive exposure to cortisol. The main cause of is prescribed glucocorticoids, but there can also be endogenous causes:
- Cushing’s Disease: caused by a benign pituitary adenoma that secretes ACTH.
- Adrenal Cushing’s: caused by excess cortisol produced by an adrenal tumour.
- Other: caused by non-pituitary-adrenal tumours producing ACTH and/or CRH (e.g. small cell lung cancer - very rare).
The signs and symptoms of Cushing’s syndrome include:
- Plethoric moon-shaped face
- Buffalo hump - hump behind the shoulders caused by fat deposition
- Abdominal obesity - due to the redistribution of fat
- Purple striae - deposition of fat stretches the skin
- Acute weight gain
- Hyperglycaemia - due to increased gluconeogenesis
- Hypertension - due to mineralocorticoid effects of excess cortisol
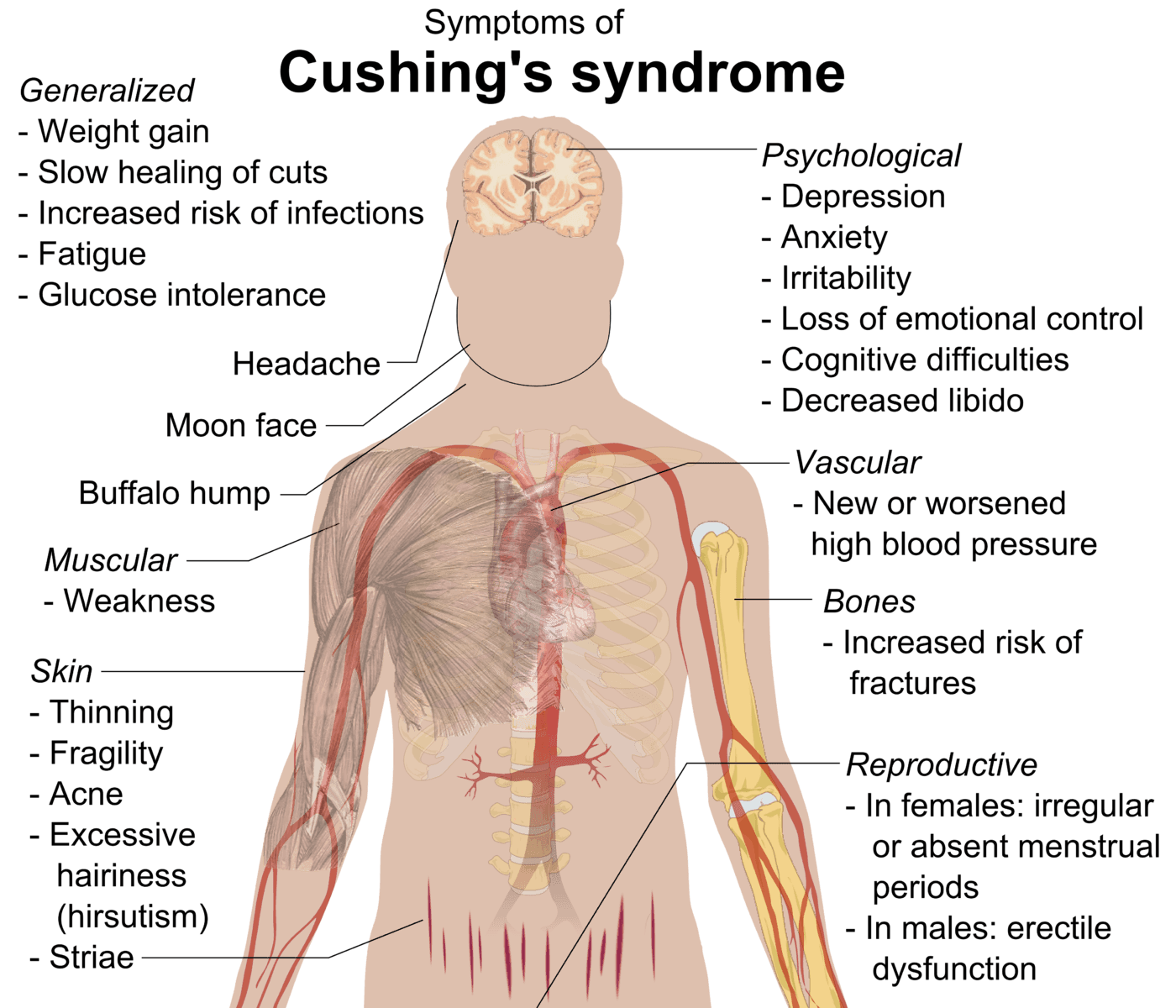
Diagram: Shows the symptoms of a patient with Cushing's Syndrome.
Creative commons source by Mikael Häggström [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
It is important to note that the side effects of steroid drugs (e.g. prednisolone and dexamethasone) are the same as the effects of higher levels of cortisol. Steroid dosage should always be reduced gradually, and not stopped suddenly because the body needs to become accustomed to the lower levels gradually.
Androgens and Genetic Defects in the Adrenal Glands
Androgens are partially regulated by ACTH and CRH.
In pre-pubescent males dehydroepiandrosterone (DHEA) released from the adrenal glands is converted to testosterone in the testes. After puberty this production is insignificant as the testes themselves release testosterone.
In females, adrenal androgens promote libido, and are converted to oestrogens by other tissues. After menopause this is the only source of oestrogens in the body. The ovaries act as a source of oestrogens after puberty.
In both sexes, androgens promote axillary and pubic hair growth.
A number of clinical conditions can arise as a genetic defect in one or more enzymes required for the synthesis of corticosteroid hormones from cholesterol. This causes a lack of cortisol, which in turn causes secretion of large amounts of ACTH (think about the impact on the negative feedback loop discussed earlier), which in turn causes adrenal cortex hyperplasia.
The severity and consequences of these conditions depend on which enzyme is affected. The most common form of genetic defect is deficiency of the 21-hydroxylase enzyme. The position of the enzyme in the biosynthetic pathway of corticosteroids means that the enzyme deficiency results in decreased glucocorticoid and mineralocorticoid production. The precursor of these hormones (17α-hydroxyprogesterone) is therefore diverted to more androgen synthesis, which can result in genital ambiguity in female infants due to the conversion of this excess androgen to testosterone, and “salt-wasting crises” due to a high rate of sodium loss in urine.
The adrenal medulla is a modified sympathetic ganglion of the autonomic nervous system which contains chromaffin cells. Chromaffin cells lack axons, but act as post-ganglionic nerve fibres that release adrenaline and noradrenaline (both catecholamines) into the blood. Adrenaline and noradrenaline are released in a 4:1 ratio (20% of chromaffin cells lack the N-methyl transferase enzyme, the enzyme needed to convert noradrenaline into adrenaline).
Adrenaline works through associated GPCR receptors, and its actions can be thought of in terms of the fight or flight response.
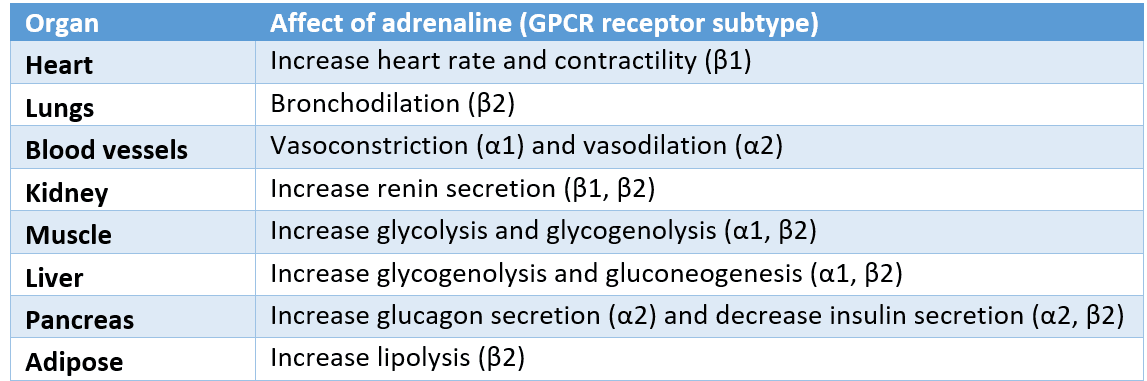
Diagram: Shows the actions of adrenaline in the body and the receptors it acts on.
SimpleMed Original by Dr. Jenny Hubball
Phaeochromocytoma
A chromaffin cell tumour that secretes catecholamines (mainly noradrenaline). Phaeochromocytomas are histiologically characteristic in that they stain dark with chromium salts.
The characteristics of the tumour are:
- Severe hypertension - this may become life-threatening
- Headaches
- Palpitations
- Diaphoresis
- Anxiety
- Weight loss
- Elevated blood glucose
The primary treatment of a phaeochromocytoma is surgical resection, but pharmacological blockade of catecholamine receptors (with alpha blockade established before any beta blockade) is an important part of management.
Addison’s disease
Chronic adrenal insufficiency. This is most commonly caused by destructive atrophy of the adrenal glands by autoimmune response. In the past, the main cause has been tuberculosis, and other, much rarer causes include fungal infection, adrenal cancer, and adrenal haemorrhage.
Signs and symptoms of Addison’s disease include:
- Postural hypotension
- Lethargy
- Weight loss
- Anorexia
- Increased skin pigmentation
- Hypoglycaemia
The hyperpigmentation seen in Addison’s disease is caused by a decrease in cortisol levels. This means there is reduced negative feedback on the anterior pituitary, and so more pro-opiomelanocortin (POMC) is produced to synthesise ACTH. POMC also produces alpha-melanocyte-stimulating hormone (α-MSH), which causes melanin synthesis, and thus hyperpigmentation. The ACTH itself can also activate melanocortin receptors on melanocytes, thus also contributing to the hyperpigmentation directly.
Addisonian crisis
A life-threatening emergency due to adrenal insufficiency.
An Addisonian crisis can be caused by:
- Severe stress
- Salt deprivation
- Infection
- Trauma
- Cold exposure
- Over exertion
- Abrupt steroid drug withdrawal
The signs and symptoms include:
- Nausea
- Vomiting
- Pyrexia
- Hypotension
- Vascular collapse
An Addisonian crisis is treated with urgent intravenous fluid replacement and high-dose intravenous hydrocortisone.
Suspected adrenocortical disease is diagnosed through the measurement of plasma cortisol and ACTH, and the measurement of 24 hr urinary excretion of cortisol and its breakdown products. In addition, dynamic function tests (e.g. the dexamethasone suppression test and ACTH stimulation tests) may be used in the differential diagnosis of adrenocortical disease.
- Dexamethasone suppression of plasma cortisol by >50% is characteristic of Cushing’s disease, but suppression doesn’t normally occur in adrenal tumours or ectopic ACTH production.
- The administration of Synacthen would normally increase plasma cortisol, and a normal response to this usually excludes Addison’s disease.
Reviewed by: Dr. Marcus Judge
Edited by: Dr. Maddie Swannack
- 12142

