Next Lesson - Carbon Dioxide Transport
Contents
- Kinetic Theory of Gases
- Boyle’s Law
- Partial Pressure of an Individual Gas in a Gas Mixture
- Partial Pressure of a Gas in a Liquid
- Content of a Gas in a Liquid
- Saturated Vapour Pressure
- Partial Pressure of Gases in the Alveoli
- Partial Pressure of Gases in the Venous System
- Gas Exchange in the Lungs
- Oxygen in the Blood
- Measuring Adequacy of Oxygenation
- Ventilation/Perfusion Ratio
- Quiz
Abstract
- Boyle’s Law states that the pressure of a gas at a constant temperature is indirectly proportional to its volume. By decreasing the volume, the number of collisions between molecules in the gas increases, meaning the pressure increases.
- In a mixture of gases, each individual gas exerts a partial pressure in proportion to its volume percentage and the total pressure is equal to the sum of the partial pressures of the individual gases.
- When a gas dissolves in a liquid, the gas molecules can collide with the wall of the container holding the liquid; this generates a pressure within the liquid, called the partial pressure of gas in a liquid. It is important when considering the dissolved gases in blood, like oxygen and carbon dioxide.
- The total content of a gas dissolved in a liquid is equal to the amount of gas dissolved in the solution plus the amount of gas reacted with a component. For oxygen, this involves the amount of oxygen dissolved in the blood and the amount of oxygen reacted with haemoglobin.
- An equilibrium exists between the rate of gas dissolving into a liquid and the amount of liquid evaporating. Once equilibrium has been reached, the gas mixture inside the airways is saturated with water vapour and there is a pressure exerted - saturated vapour pressure.
- The partial pressure of oxygen in the alveolus is determined by two factors: the rate at which oxygen diffuses into the blood, and the rate at which oxygen is replenished by ventilation in the alveoli.
- The rate at which oxygen diffuses into the blood and carbon dioxide out of the blood is determined by three factors: the space available for gas exchange, the barriers to diffusion, and the gradient of partial pressures.
- Haemoglobin transports oxygen from the lungs to the peripheral tissues and returns carbon dioxide to the lungs from the tissues. Myoglobin acts as an intracellular storage of oxygen in muscles.
- There are four factors that reduce haemoglobin’s affinity for oxygen: high partial pressure of carbon dioxide, low pH of the blood, increased temperature, and high concentrations of 2,3-BPG.
- A patient’s adequacy of oxygenation can be measured through two measures: arterial blood gas and peripheral oxygen saturation (pulse oximetry).
- For continuous delivery of oxygen and removal of carbon dioxide from the lungs, it is very important that ventilation and perfusion are matched. The ventilation-perfusion ratio can become mismatched through poor ventilation or poor perfusion. A mismatch leads to hypoxaemia, which stimulates hyperventilation to try to increase the oxygen saturation of the blood. In the lungs, blood is diverted away from the poorly ventilated/perfused regions to healthier areas of the lungs through the process of hypoxic vasoconstriction.
Core
Gases are molecules that move randomly in a space, and pressure is generated when these molecules collide with a surface. If these collisions occur more frequently, then the pressure is higher.
Boyle’s Law states that the pressure of a fixed quantity of gas at a constant temperature (e.g. body temperature) is indirectly proportional to its volume. By decreasing the volume, the number of collisions between gas molecules increases, and so does the pressure.
In the lungs, as the volume of the thoracic cavity increases during inspiration, the number of collisions of molecules of gases decreases, meaning the pressure decreases below the atmospheric pressure. This allows gas from outside the lungs to move into the thoracic cavity down a pressure gradient.
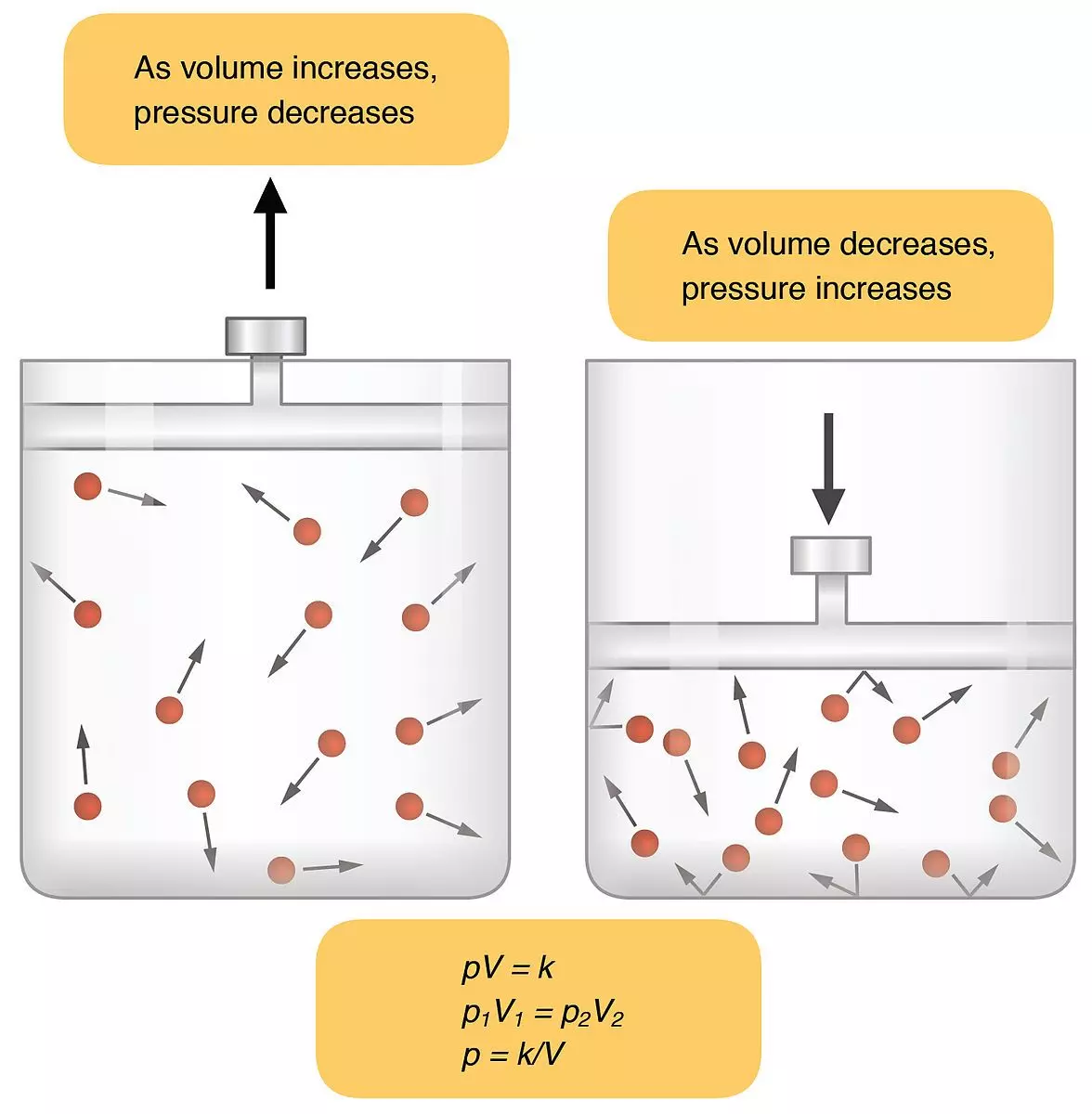
Diagram - Demonstration of Boyle’s Law
Creative commons source by OpenStax College [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
Partial Pressure of an Individual Gas in a Gas Mixture
When there is a mixture of gases, such as in the atmosphere, each individual gas exerts a partial pressure in proportion to its fractional concentration in the mixture.
Therefore, the total pressure is equal to the sum of the partial pressures of the individual gases.
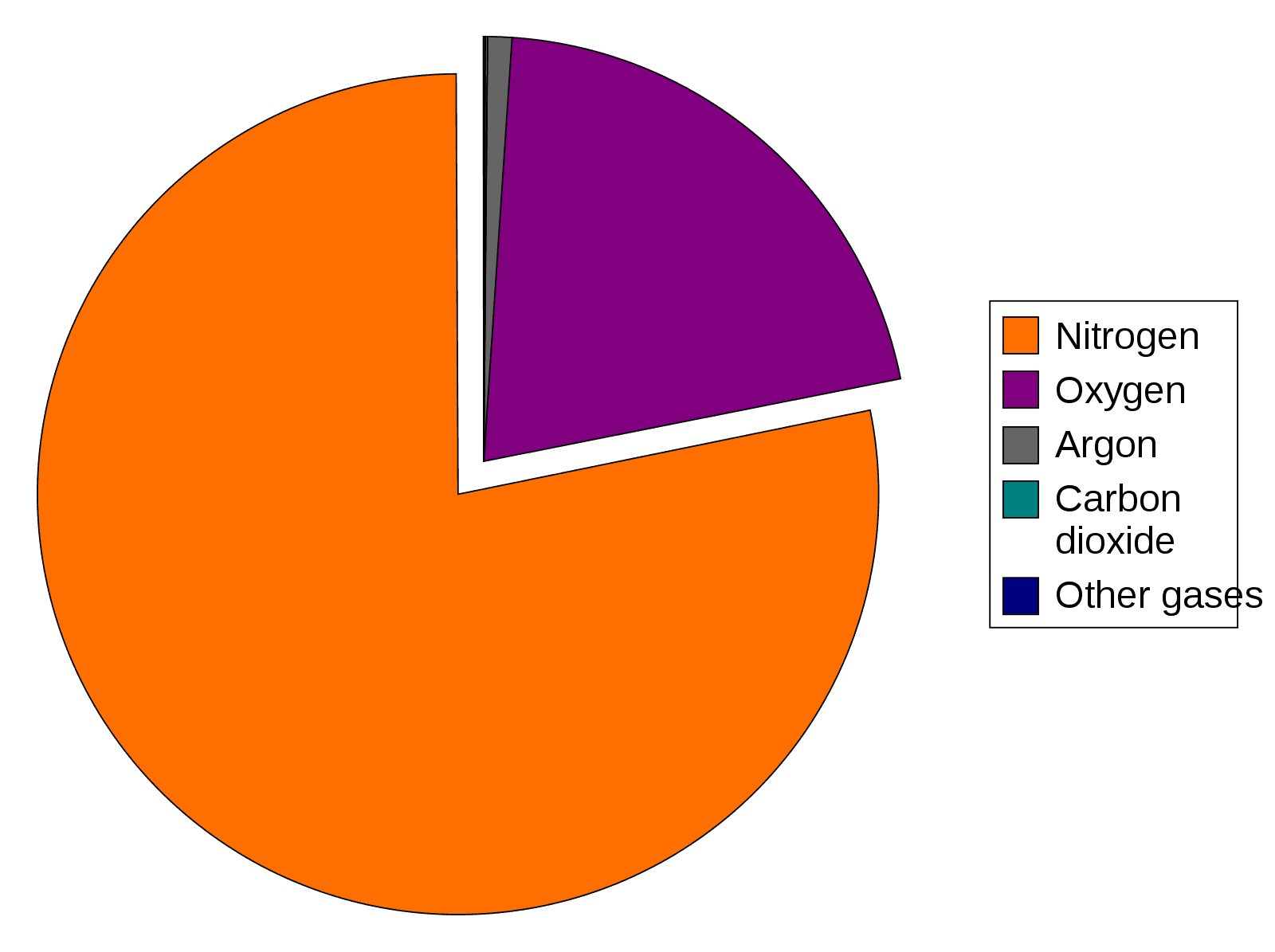
Image - The composition of Earth's atmosphere
Public Domain Source by Dbc334 [Public domain]
The atmospheric pressure is 101 kPa and air contains 20.9% of oxygen, the partial pressure of oxygen (pO2) in atmospheric air is 101 x 0.209 = 21.1kPa. The other ~80kPa is made from the partial pressures of other gases in the atmosphere, as shown in the diagram above.
At higher altitudes, the atmospheric pressure is lower, meaning the molecules of gases are further apart, so there are fewer oxygen molecules available for us to breathe. This helps explain why many climbers use supplemental oxygen when ascending very high altitudes such as Mount Everest.
Gases move down a pressure gradient from high partial pressure to low partial pressure; this explains why oxygen moves from the alveolus (high) into the blood (low).
Partial Pressure of a Gas in a Liquid
When a gas comes into contact with a liquid, the gas molecules dissolve into the liquid. Some of the molecules already in the liquid will evaporate, becoming gases. When the rate of the gas entering the liquid is equal to the rate of gas leaving the liquid, the system is said to be in equilibrium.
Once the gas has dissolved in the liquid, the gas molecules can collide with the wall of the container and generate a pressure within the liquid. This is known as the partial pressure of gas in a liquid.
This is important to medicine because in the alveoli, the partial pressure of oxygen is about 13.3 kPa. This ability of oxygen to diffuse into a liquid and form an equilibrium means that the partial pressure of oxygen in blood leaving the lungs approaches this value.
The amount of a gas dissolved in the liquid and the partial pressure of a gas are two different measurements.
The content of a gas in a liquid is equal to the amount of gas dissolved in solution (the partial pressure of gas in a liquid) plus the amount of gas reacted with a component.
To calculate the amount of the gas dissolved, multiply the partial pressure of the gas in the gas phase and the solubility coefficient of the gas together. The solubility coefficient of an individual gas is constant and is defined as the amount of a gas that will dissolve in a litre of plasma at 37oC upon exposure to a given partial pressure.
As an example, for oxygen the solubility coefficient = 0.01mmol/L. When a litre of plasma at 37oC is exposed to alveolar air with a pO2 of 13.3 kPa, the oxygen content of the plasma will be 0.13mmol/L (13.3 x 0.01).
Oxygen can also be bound to haemoglobin in the blood to be transported around the body, meaning that the content of oxygen in blood = amount of oxygen bound to haemoglobin AND amount of gas dissolved in the blood.
Normal Hb concentration is around 2.2mmol/L and each molecule of haemoglobin can bind to four oxygen molecules, therefore the amount bound to haemoglobin is 8.8mmol/L. Overall, the total oxygen content is 8.93mmol/L (8.8 + 0.13). This shows why it is important to have haemoglobin molecules in the blood, as they are responsible for most of the oxygen transport in the blood.
Once oxygen has dissolved in the blood, it can enter red blood cells to bind with haemoglobin molecules. After the haemoglobin molecule is fully saturated with four oxygen molecules, oxygen can continue to dissolve into the blood until the pO2 of plasma equals pO2 of alveolar air.
When gas molecules encounter water in the airways they dissolve, and water molecules evaporate to enter the air. An equilibrium is set up when the rate of evaporation is equal to the rate of water vapour returning to the liquid phase. Once equilibrium has been reached, the gas mixture inside the airways is saturated with water vapour and there is a pressure exerted by the water vapour. This pressure is called saturated vapour pressure and it is dependent on body temperature.
The total pressure in the airways is still equal to atmospheric pressure (101kPa). However, in the airways with the equilibrium of water evaporation and condensing taking place, water contributes 6.28kPa of pressure. This means that the other gases make up the remainder: 94.7kPa (101 - 6.28). The other gases are in the same proportion in the lungs as in the atmosphere meaning the pO2 of the humidified air in the airways = 19.8kPa (94.7 x 0.209).
This means that the partial pressure of oxygen changes as it moves from the nostrils to the alveoli. In the nostrils and upper respiratory tract, the inhaled air becomes humidified.
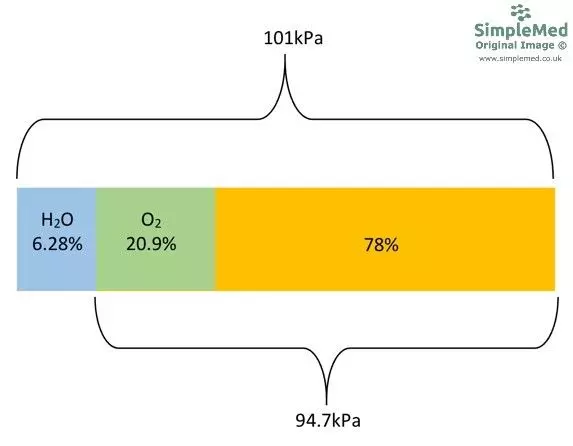
Diagram - The distribution of gas and water partial pressure
SimpleMed original by Dr. Peter Parkinson
Partial Pressure of Gases in the Alveoli
The partial pressure of oxygen (pO2) in the alveolus is determined by two factors: the rate at which oxygen diffuses into the blood and the rate at which oxygen is replenished by ventilation. The ideal pO2 in the alveoli is around 13.3kPa and it is the balance between perfusion and ventilation that maintains this value. If a person hyper- or hypoventilates, the pO2 in the alveolar gas will change.
The normal value for the partial pressure of carbon dioxide (pCO2) in the alveoli is 5.3kPa; this is determined by the rate at which CO2 enters the alveoli from the blood and the rate at which CO2 is breathed out. Again, if a person hyper- or hypoventilates, the pCO2 will change.
Partial Pressure of Gases in the Venous System
Venous blood that reaches the pulmonary capillaries has a pO2 of 6kPa and pCO2 of 6kPa. At the alveolar capillary membrane, gas exchange takes place and the blood equilibrates with the alveolar air, meaning the blood leaving the lungs for the heart has a pO2 of 13.3kPa and a pCO2 of 5.3kPa. This change is what allows the lungs to supply the body with oxygen and to get rid of the carbon dioxide produced.
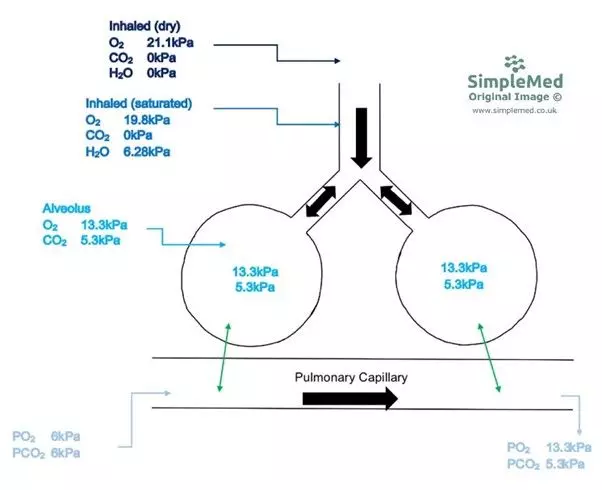
Diagram - The partial pressures in the respiratory tract and the pulmonary capillaries
SimpleMed original by Dr. Peter Parkinson
The rate at which oxygen and carbon dioxide diffuse between the blood and the alveolar space is determined by three factors:
1. Area Available for Gaseous Exchange
The area for gas exchange is around 70m2 and is formed by the large alveolar surface and the huge number of alveoli in the lungs.
This can be affected by lung diseases like emphysema as there is destruction of the alveolar walls and this reduces the total surface area available for gas exchange.
2. Diffusion Barriers
Even though the distance between the alveolar gas and the alveolar capillary blood is short, there are a number of structures between the two. These structures are:
- Alveolar epithelial cell
- Interstitial fluid
- Capillary endothelial cell
- Plasma
- Red cell membrane
Gas exchange occurs primarily across the alveolar-capillary membrane, which consists of a single layer of alveolar epithelium and a single layer of capillary endothelium.
The resistance to diffusion is not the same for oxygen and carbon dioxide; the rate of diffusion for the gases is affected by the solubility of the gases in water. Carbon dioxide is more soluble than oxygen and therefore diffuses across the barriers at a faster rate. As a result, oxygen diffusion is affected more in diseased states such as interstitial lung disease, where there is excessive deposition of collagen and extracellular matrix in the interstitium. This process increases the distance of the diffusion pathway and thickens the walls of alveoli. Conditions like pulmonary oedema also increase the diffusion pathway because there is fluid in the alveolus.
3. Gradient of Partial Pressure
To allow carbon dioxide to diffuse out of the blood, and oxygen to diffuse in, a steep concentration gradient is beneficial. This means that the steep difference between the alveolar partial pressures (13.3kPa for oxygen and 5.3kPa for carbon dioxide) and the blood arriving at the lungs (approximately 6kPa for oxygen and 6kPa for carbon dioxide) is helpful for gas exchange.
Oxygen is not soluble enough in blood for this to be the only mechanism of transport around the body. This means that an alternative mechanism of oxygen transport is needed. The protein, haemoglobin, found in red blood cells reversibly loads and unloads oxygen within a very narrow range of partial pressures of oxygen. Myoglobin is only found in muscle tissue and acts as a storage molecule by only unloading oxygen at very low partial pressures of oxygen e.g. anaerobic conditions.
Myoglobin is a haem protein and is monomeric (single polypeptide chain). One myoglobin molecule comprises one haem group that contains an iron ion (Fe2+), which binds with an oxygen molecule to transport the oxygen in the blood.
Myoglobin is only found in muscle tissue and acts as a storage molecule for oxygen, which then can be released in times of oxygen deprivation. When oxygen is in high demand (like when exercising), the oxygen can be released from myoglobin and can allow the muscle cells to carry on generating energy.
Myoglobin binds firmly and tightly to oxygen molecules and because of this, produces a hyperbolic curve on an oxygen dissociation curve.
The graph below shows this hyperbolic curve when plotting the saturation of myoglobin against increasing partial pressures of oxygen: it shows that myoglobin has a high affinity for oxygen because the partial pressure of oxygen needed to occupy 50% of the myoglobin molecules is very low (P50 - shown in red on this graph).
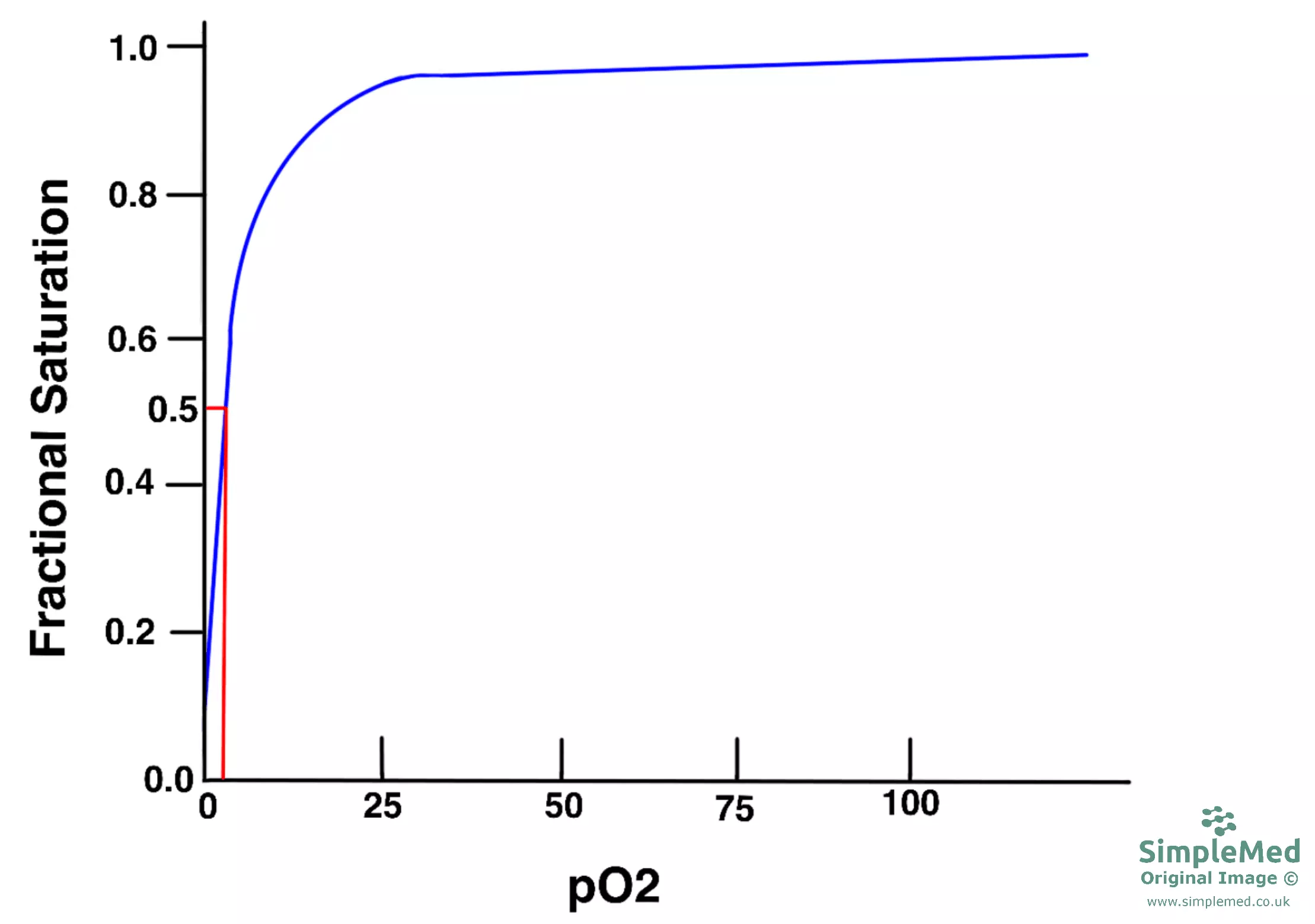
Diagram - Myoglobin oxygen dissociation curve and the P50 of myoglobin (red line)
SimpleMed original by Dr. Peter Parkinson
Haemoglobin like myoglobin is a haem protein but is made up of four subunits. Each subunit contains a haem group containing an iron ion, which binds with the oxygen molecules. This means that each haemoglobin molecule can bind with a maximum of four oxygen molecules.
Because haemoglobin has four polypeptides making up its quaternary protein structure, the binding of oxygen can cause changes within the structure of the molecule. Each haemoglobin molecule can exist in a tense (T) or a relaxed (R) state; when no oxygen molecules are bound to haemoglobin, it exists in the T state, making it difficult for the first oxygen to bind. After one oxygen molecule binds to a haem group, the haem molecule stabilises in the R conformation making it easier for the subsequent oxygen molecules to bind.
The relationship between oxygen and haemoglobin can be described using the haemoglobin-oxygen dissociation curve. The shape of the haemoglobin-oxygen disassociation curve is sigmoidal, due to the cooperative binding of oxygen; difficult for the first oxygen molecule to bind due to the low affinity for oxygen in the T state, but binding becomes easier for subsequent oxygen molecules because haemoglobin molecules stabilise in the R state.
It is also important to understand why the oxygen-haemoglobin dissociation curve flattens off at high pO2. This just occurs due to the increasing number of saturated haemoglobin subunits - it takes the oxygen more time to find a free binding site.
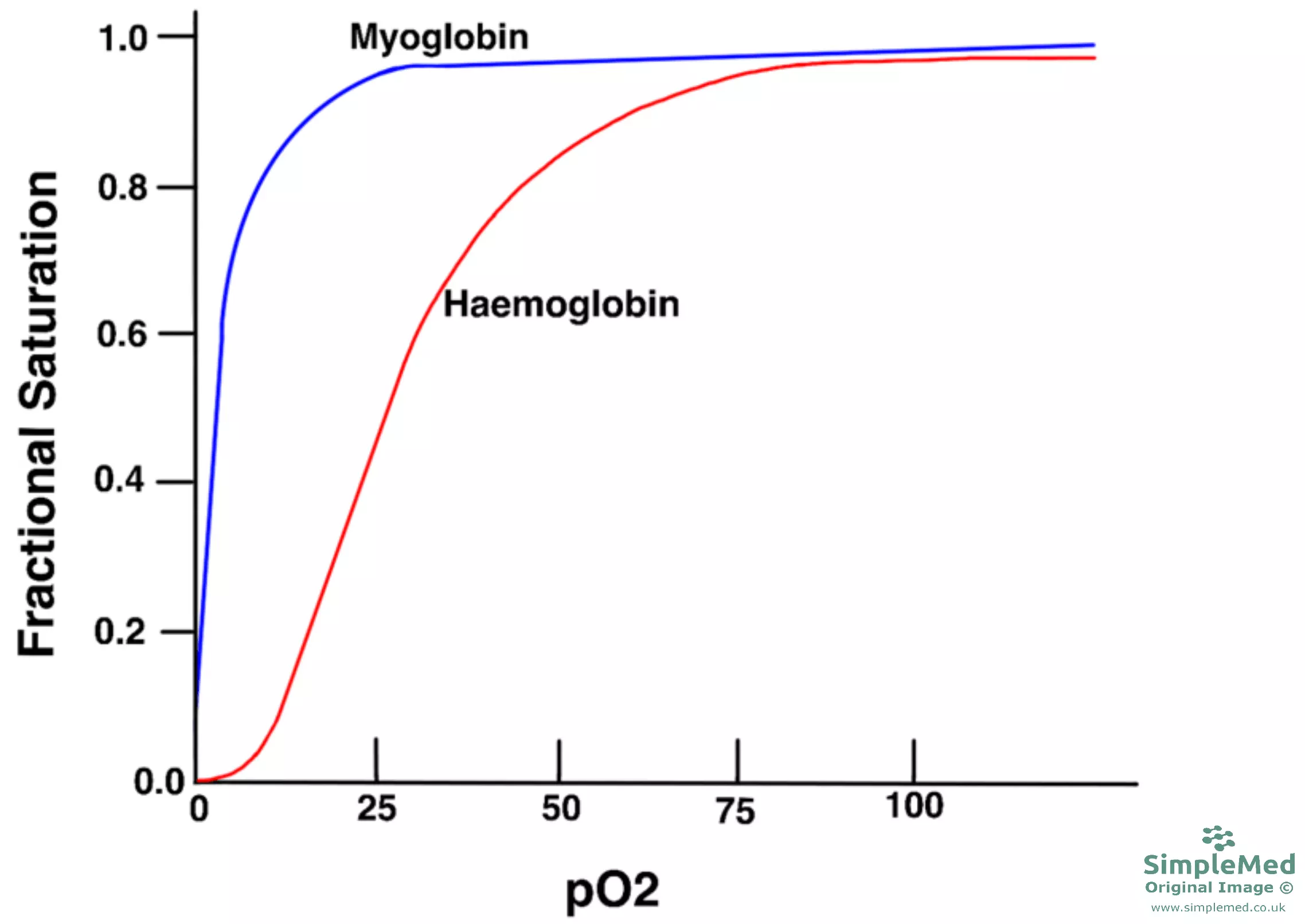
Diagram - Haemoglobin and myoglobin oxygen disassociation curve
SimpleMed original by Dr. Peter Parkinson
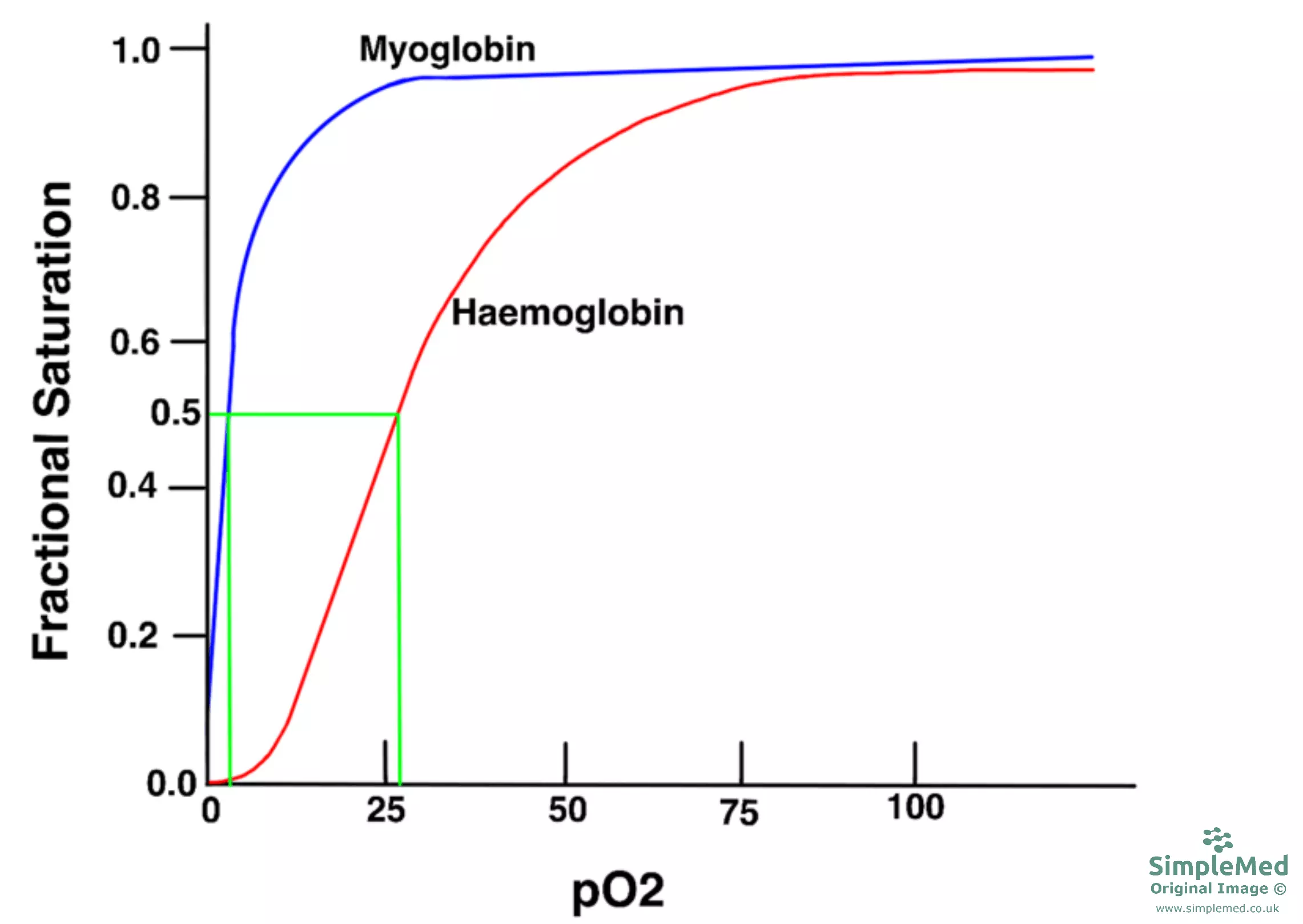
Diagram - P50 of myoglobin in comparison to haemoglobin
SimpleMed original by Dr. Peter Parkinson
Once the blood leaves the lungs, it is carrying nearly all the oxygen it can and has the pO2 of 13.3kPa (equal to the pO2 of the alveolar gas). Once it gets to the tissues, there is an increased amount of CO2 diffusing into the blood. This causes a fall in the pH of the blood (because CO2 reacts with H2O to form H+ and HCO3-), and this change in pH encourages haemoglobin into the T state. As explained earlier, the T state is not ideal for oxygen binding as it lowers the affinity of haemoglobin for oxygen, meaning that the haemoglobin molecule releases the oxygen bound into the blood so that it can diffuse into the tissues.
In actively respiring tissues, there is a large production of carbon dioxide through increased metabolism and this will lower the pH of the local blood. This causes haemoglobin’s affinity for oxygen to decrease, meaning more oxygen will be provided to actively respiring tissues through increased unloading.
The overall physiological effect of carbon dioxide acting to decrease the pH and cause a decreased affinity of haemoglobin for oxygen is called the Bohr effect. This effect enhances the unloading of oxygen through metabolically active tissues and ensures the delivery of oxygen meets the demands of the tissues.
The Bohr effect will shift the oxygen dissociation curve to the right, increasing the P50 of haemoglobin, therefore, reducing the affinity of haemoglobin for oxygen. A good way to remember the direction of the shift is that the BohR shift moves the curve Right.
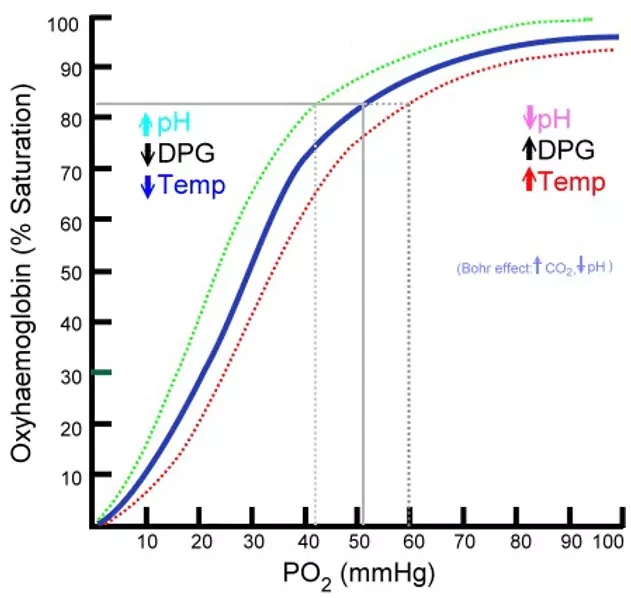
Diagram - The Bohr effect
Public Domain Source by Ratznium, edited by Dr. Peter Parkinson [Public domain]
Increased temperature can also shift the oxygen-haemoglobin curve to the right, because heat encourages haemoglobin into the T state, meaning in metabolically active tissues where the temperature is slightly higher increased amounts of oxygen are disassociated.
2, 3-bisphosphoglycerate (2,3-BPG)
2,3-BPG is a molecule in the blood that acts as an allosteric effector to haemoglobin. It can bind to the positively charged amino acid residues in the centre of the haemoglobin structure, encouraging stabilisation of haemoglobin in the T state. By binding to haemoglobin, 2,3-BPG lowers the affinity haemoglobin has for oxygen, meaning high concentrations of 2,3-BPG increase the amount of oxygen released in peripheral tissues.
There are situations where a person may have a higher than expected level of 2,3-BPG due to low tissue oxygen concentrations. This is beneficial because it encourages more oxygen to dissociate at the peripheral tissues. This may occur if the person has acclimatised to living at higher altitudes (where inspired pO2 is lower anyway) and if a person is suffering from congestive heart failure (blood flow to peripheral tissues is reduced).
Measuring Adequacy of Oxygenation
Oxygenation can be measured by either a pulse oximeter or by taking an arterial blood gas.
The patient’s oxygen saturation can be measured by using a pulse oximeter, a non-invasive, quick technique that can monitor the percentage of haemoglobin saturated with oxygen. The principle of the instrument is based on that oxygenated haemoglobin and deoxygenated haemoglobin absorb different wavelengths of light.
It does have a number of downfalls: if a patient is very unwell or has poor circulation to their peripheries, it will not get an accurate measurement, and it can also be misleading in severe anaemia, as oxygen saturation may appear normal despite a reduced oxygen content due to low haemoglobin.
A sample of blood from an artery is taken, usually from the radial artery in the forearm, and analysed into a machine to assess the pO2, pCO2, pH, and concentration of certain ions. These parameters can be very helpful when assessing the function of the patient’s lungs and kidneys. However, taking a blood sample from an artery can be painful and difficult to perform, so this is reserved for patients who are quite unwell.
To get enough oxygen to the peripheral tissues and to exhale all the waste carbon dioxide, the ventilation and the blood supply of the alveoli need to be well matched.
To calculate the ventilation rate (V), multiply the tidal volume by the respiratory rate, which gives the minute ventilation. For the average adult (70 kg), minute ventilation is around 6 L/min, while alveolar ventilation is lower after accounting for dead space, at approximately 4.2 L/min.
Perfusion (Q) of the lungs is defined as the total volume of blood that reaches the pulmonary capillaries in a given period of time.
The V/Q ratio ideally would be 1 because this would mean that the most efficient diffusion of oxygen and carbon dioxide was taking place. In reality, due to some areas of the lungs being ventilated less well (like the hilar region where there are many conducting airways but few alveoli), the actual V/Q ratio is closer to 0.8.
Ventilation-perfusion mismatch is the most common cause of hypoxaemia (low concentration of oxygen in the blood).
V/Q mismatch can occur due to reduced ventilation of regions of the lungs. There are some disorders that can result in poor ventilation to a region of alveoli, and these include:
- Early Stages of Asthma - airway narrowing secondary to smooth muscle contraction makes it more difficult to ventilate the alveoli.
- Early Stages of COPD - airway narrowing due to inflammation and loss of radial traction makes it harder to ventilate alveoli.
- Pneumonia - exudate found in the alveoli increases diffusion distance and impairs delivery of oxygen to the alveolar membrane.
- Respiratory Distress Syndrome in Neonates - alveoli are less compliant, meaning ventilation takes a lot of effort.
The poorly ventilated alveoli will have a V/Q ratio of < 1 which results in a decrease in pO2 of the affected alveoli. This low pO2 prompts hyperventilation, causing pCO2 to remain normal as the increased carbon dioxide is blown off due to hyperventilation.
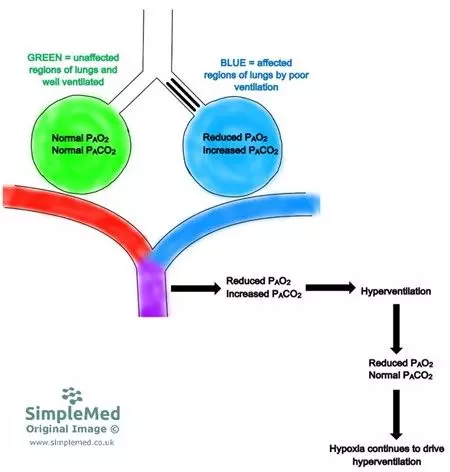
Diagram - Ventilation-perfusion mismatch due to poor ventilation
SimpleMed original by Dr. Peter Parkinson
V/Q mismatch can also occur due to reduced perfusion to regions of the lungs. Perfusion to areas of the lungs can be reduced by a pulmonary embolus, a blood clot blocking a branch of the pulmonary artery. Ventilation to the region of the lung affected by the embolus is wasted as no gas exchange can occur there. This results in a V/Q ratio of <1.
The body compensates by diverting blood away from poorly ventilated regions of the lungs to better ventilated areas through a process called ‘hypoxic vasoconstriction’. In pulmonary embolism, perfusion is mechanically reduced, so this compensatory mechanism is limited. This means that perfusion is increased to these functional regions. However, the blood that is supplying the functional regions is already saturated with oxygen (as is normal for any area of the lungs) meaning it cannot saturate further. The low pO2 of the blood leaving the lungs drives hyperventilation, which results in a low or normal pCO2 of the blood leaving the lungs.
Edited by: Dr. Maddie Swannack
Reviewed by: Dr. Thomas Burnell
- 9423

